Notice Lecture notes in power point format will














- Slides: 24

Notice • Lecture notes in power point format will be posed on COLA ftp site after the lecture is given. • The site for download is ftp: //grads. iges. org/pub/huangb/fall 2004 • Hard copies of the notes will still be provided in class.

Ocean dimensions and Shapes

Geography The oceans are basins in the surface of the solid earth containing salt water • Major ocean areas The Southern Ocean (south of 30 o-40 o. S) The Atlantic Ocean The Pacific Ocean The Indian Ocean The Arctic Sea (These regions are distinguished in terms of land masses (last four) and oceanographic characteristics (circulations, the Southern Ocean) • Other smaller water basin (seas bounded by land or island chains) Mediterranean Sea The Caribbean Sea The Sea of Japan The Bering Sea, etc. • Areas of open oceans are sometimes also referred to as "seas", mainly for historical reasons and geographical convenience (examples: Greenland, Norwegian, Iceland, Labrador, Weddell, Ross, Arabian Seas, Bay of Bengal etc. )

The scales of the major oceans • Percentage to total ocean area and zonal scales Oceans Pacific Atlantic Percentage* zonal scale 46% 15, 000 km 23% 5, 000 km Indian 20% 5, 000 km *neighboring sectors of the Southern Ocean included • Pacific is as large as the Atlantic and Indian Ocean combined

Basic Ocean-Land Comparison • Percentage of earth covered by sea (71%) and by land (29%). Land-sea ratio: Southern hemisphere (4: 1) Northern hemisphere (1. 5: 1) • The oceans are much deeper than the land is high The average ocean depth: ~4000 meters (3730 m) (the ratio of the horizontal and vertical scales are very large generally scaled by 1/1000 in vertical sections) The average land elevation: 840 m (The smaller seas are generally about 1200 m deep or less) 84% sea bottom is more than 2000 m deep (11% land surface is more than 2000 m) • Maximum depth in the oceans: western Pacific Mindanao Trench (11, 524 m) • Maximum height on land: Mt. Everest: 8840 m

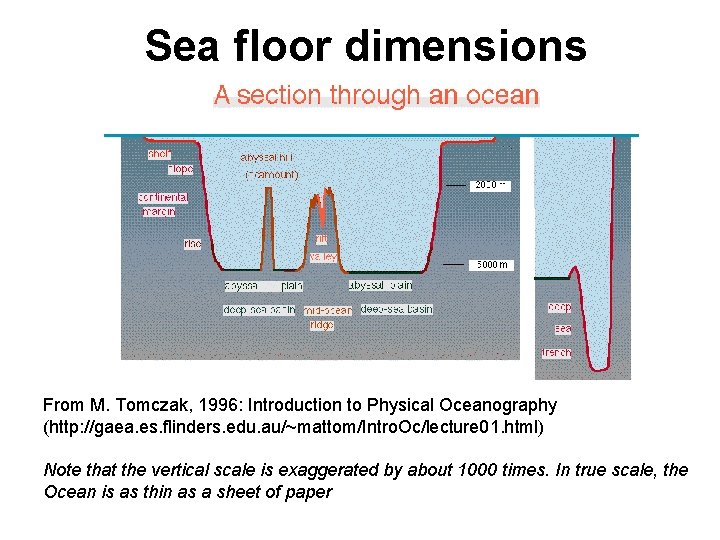
Sea floor dimensions From M. Tomczak, 1996: Introduction to Physical Oceanography (http: //gaea. es. flinders. edu. au/~mattom/Intro. Oc/lecture 01. html) Note that the vertical scale is exaggerated by about 1000 times. In true scale, the Ocean is as thin as a sheet of paper

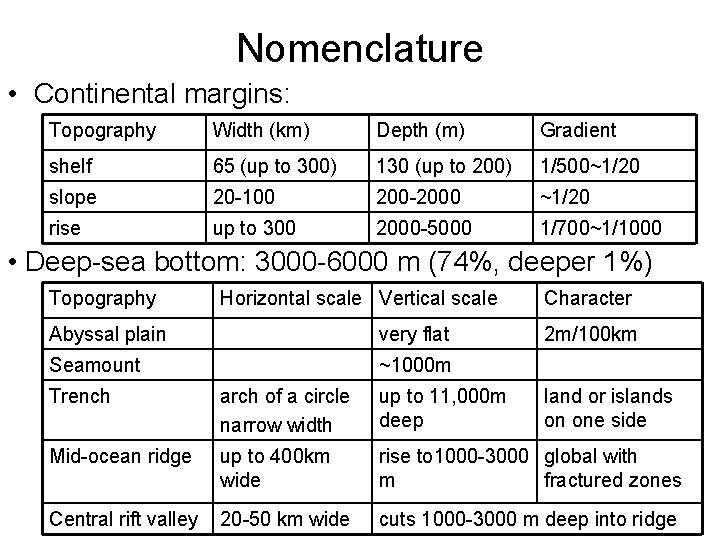
Nomenclature • Continental margins: Topography Width (km) Depth (m) Gradient shelf 65 (up to 300) 130 (up to 200) 1/500~1/20 slope 20 -100 200 -2000 ~1/20 rise up to 300 2000 -5000 1/700~1/1000 • Deep-sea bottom: 3000 -6000 m (74%, deeper 1%) Topography Horizontal scale Vertical scale Abyssal plain very flat Seamount ~1000 m Character 2 m/100 km Trench arch of a circle narrow width up to 11, 000 m deep land or islands on one side Mid-ocean ridge up to 400 km wide rise to 1000 -3000 global with m fractured zones Central rift valley 20 -50 km wide cuts 1000 -3000 m deep into ridge

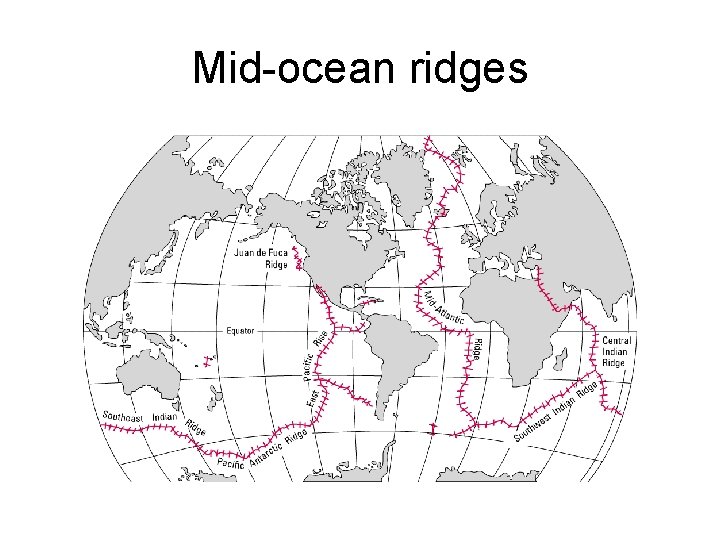
Mid-ocean ridges

Physical Properties of Sea Water • • Property of pure water Temperature Salinity Pressure Density Equation of the state Potential temperature Static Stability

Property of pure water • Sea water is a mixture of 96. 5% pure water and 3. 5% other material, such as salts, dissolved gases, organic substances, and undissolved particles. • Many physical properties of see waters are determined by the 96. 5% pure water.

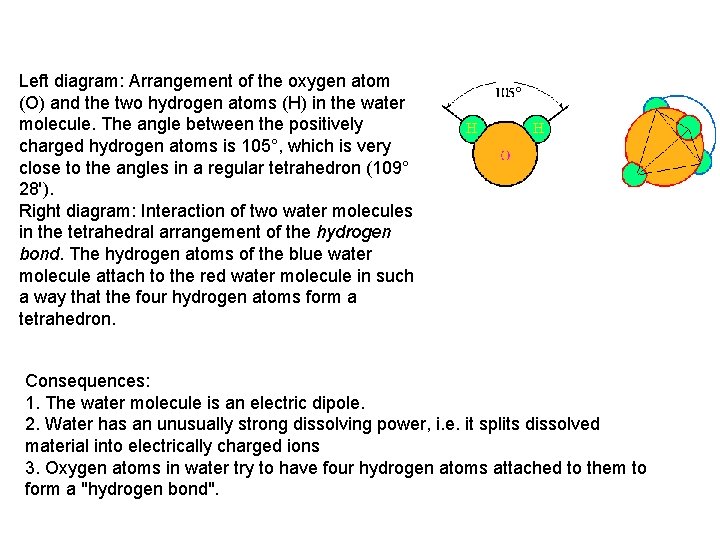
Left diagram: Arrangement of the oxygen atom (O) and the two hydrogen atoms (H) in the water molecule. The angle between the positively charged hydrogen atoms is 105°, which is very close to the angles in a regular tetrahedron (109° 28'). Right diagram: Interaction of two water molecules in the tetrahedral arrangement of the hydrogen bond. The hydrogen atoms of the blue water molecule attach to the red water molecule in such a way that the four hydrogen atoms form a tetrahedron. Consequences: 1. The water molecule is an electric dipole. 2. Water has an unusually strong dissolving power, i. e. it splits dissolved material into electrically charged ions 3. Oxygen atoms in water try to have four hydrogen atoms attached to them to form a "hydrogen bond".

Density decreases as the freezing point is approached. (Ice floats). Freezing point decreases under pressure. (Melting occurs at the base of glaciers) Water molecules form aggregates of single, two, four and eight molecules. At high temperatures the one and two molecule aggregates dominate; as the temperature falls the larger clusters begin to dominate. The larger clusters occupy less space than the same number of molecules in smaller clusters. As a result, the density of water shows a maximum at 4°C.

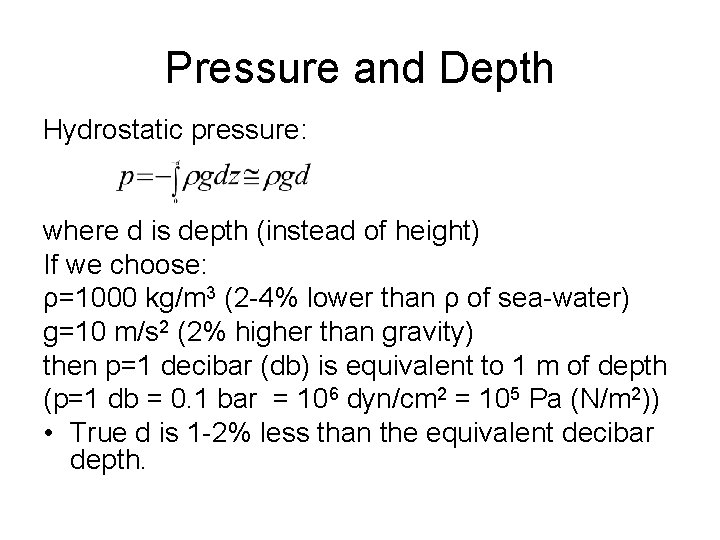
Pressure and Depth Hydrostatic pressure: where d is depth (instead of height) If we choose: ρ=1000 kg/m 3 (2 -4% lower than ρ of sea-water) g=10 m/s 2 (2% higher than gravity) then p=1 decibar (db) is equivalent to 1 m of depth (p=1 db = 0. 1 bar = 106 dyn/cm 2 = 105 Pa (N/m 2)) • True d is 1 -2% less than the equivalent decibar depth.

Temperature: (T or t) • Unit: Celsius scale (o. C) in oceanography (sometimes Kelvin, K) • Ocean range: -2 o. C to 30 o. C • Primary parameter determining density. especially in mid - and lower latitude upper ocean • Major factor in influencing the atmosphere at the surface • Temperature profile provides information on circulation features and sound speed distribution • easier to measure than other oceanic properties

Temperature Measurement Sea Surface temperature (SST) • • Bucket-sample (mercury thermometer) Radiation thermometer Subsurface temperature Nansen bottle • Protected reversing thermometer (± 0. 02 K in routine use) • in situ pressure with unprotected reversing thermometer (± 0. 5% or ± 5 m) • only a finite number (<25) of vertical points once (Mechanical) Bathythermograph (MBT) • Continuous temperature against depth (range, 60, 140 or 270 m) • Need calibration, T less accurate than thermometer (± 0. 2 K, ± 2 m) Expendable bathythermograph (XBT) • Expendeble thermister casing • dropped from ship of opportunity and circling aircraft • Graph of temperature against depth • Range of measurement: 200 to 800 m • depth is estimated from lapsed time and known falling rate

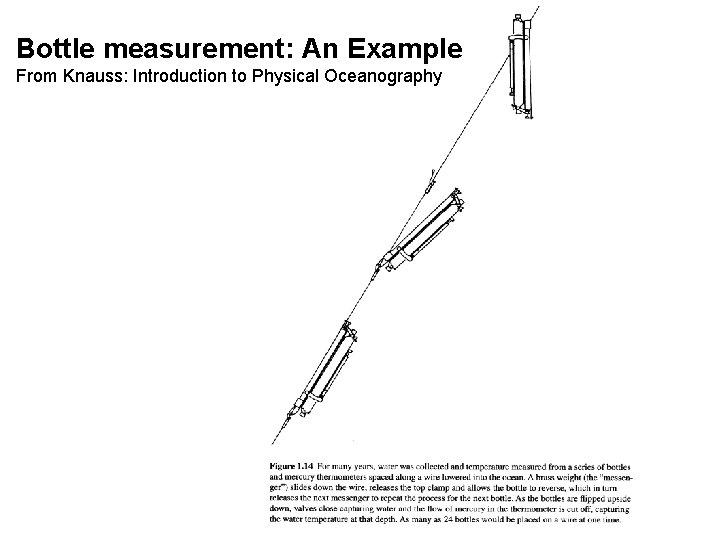
Bottle measurement: An Example From Knauss: Introduction to Physical Oceanography

Expendable bathythermograph (XBT)

Salinity is the total amount of solid materials in grams dissolved in one kilogram of sea water On average, there is around 35 gram salt in a kilogram of sea water, 35 g/kg, written as S=35‰ or S=35 ppt, read as “thirty-five parts per thousand” Globally, S can go from near 0 (coast) to about 40 ppt (Red Sea), BUT 90% is between 34 -35 ppt The most abundant ions in sea water: chlorine Cl 55. 0% sodium Na+ 30. 6% sulphate SO 4++ 7. 7% magnesium Mg++ 3. 7% potassium K+ 1. 1%

Basic properties of salt in sea water 1). The ratio of the more abundant components remain almost constant. (ocean is very well mixed globally over geological time) 2). There are significant differences in total concentration of the dissolved salts from place to place and at different depths. (There are oceanic processes continually concentrate and dilute salt in sea water in specific localities) 3). The presence of salts influences most physical properties of sea water (density, compressibility, freezing point). 4). The conductivity of the sea water is partly determined by the amount of salt it contains.

Salinity measurement Total resolved material is hard to measure routinely in seawater (e. g. , evaporation of sea-water sample to dryness) In practice, some properties of sea water are used to determine salinity. Method #1: Salinity is determined by measurements of a substitution quantity since it is contributed by its components in a fixed ratio. Method #2: Salinity is inferred from measurements of sea water’s electrical conductivity.

Absolute salinity SA Salinity can be determined through its most important component, chloride ion (plus the chlorine equivalent of the bromine and iodine), called as chlorinity (in 1902). Chlorinity is measured using titration method The relationship between salinity and chloride is based on laboratory measurements of sea water samples from all regions of the world ocean and was given in 1969 by UNESCO as SA (‰) = 1. 80655×Chlorinity (‰) SA is called as “Absolute Salinity”, unit: ppt

Practical salinity S • The practical salinity, symbol S, of a sample of sea water, is defined in terms of the ratio K of the electrical conductivity of a sea water sample of 15°C and the pressure of one standard atmosphere, to that of a potassium chloride (KCl) solution, in which the mass fraction of KCl is 0. 0324356, at the same temperature and pressure. The K value exactly equal to one corresponds, by definition, to a practical salinity equal to 35. • The corresponding formula is: S = 0. 0080 - 0. 1692 K 1/2 + 25. 3853 K + 14. 0941 K 3/2 - 7. 0261 K 2 + 2. 7081 K 5/2 • Note that in this definition, S is a ratio and is non-dimensional, but salinity of 35‰ corresponds to a value of 35 in the practical salinity. • S is sometimes given the unit “psu” (practical salinity unit) in literature (probably unnecessarily)

Salinity measurement method Knudsen (Titration) method (precision ± 0. 02) • time consuming and not convenient on board ship • not accurate enough to identify deep ocean water mass Electrical conductivity method (precision ± 0. 003~± 0. 001) • Conductivity depends on the number of dissolved ions per volume (i. e. salinity) and the mobility of the ions (ie temperature and pressure). Its units are m. S/cm (milli-Siemens per centimetre). • Conductivity increases by the same amount with ΔS~0. 01, ΔT~ 0. 01°C, and Δz~ 20 m. • The conductivity-density relation is closer than density-chlorinity • The density and conductivity is determined by the total weight of the dissolved substance

conductivity-temperature-depth probe In situ CTD precision: ΔS~± 0. 005 ΔT ± 0. 005 K Δz~± 0. 15%×z The vertical resolution is high CTD sensors should be calibrated (with bottle samples)