Notes One Unit Six Chapter 13 Solutions Definitions














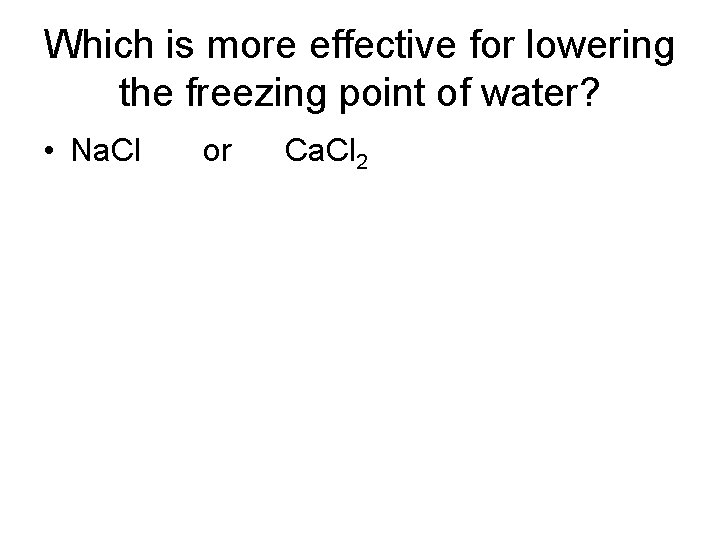




- Slides: 63

Notes One Unit Six– Chapter 13 Solutions • Definitions Pages 466 -486 • Types of Mixtures • Example Solutions • Factors Affecting Solubility • Like Dissolves Like • Solubility of Solids Changes with Temperature • Solubility of Gases Changes with Temperature • Pressure Factor • Molar Concentration • Finding Molarity From Mass and Volume • Finding Mass from Molarity and Volume • Finding Volume from Molarity and Mass

Definitions • Solutions are homogeneous mixtures. • Uniform throughout. • Solvent. • Determines the state of solution • Largest component • Solute. • Dissolved in solvent

Common Mixtures SOLUTE SOLVENT Type EXAMPLE liquid mayonnaise liquid emulsion gas liquid foam whipped cream solid dust in air gas aerosol liquid hair spray gas aerosol solid ruby glass solid liquid pearl solid emulsion gas solid foam Styrofoam

Solution Types SOLUTE SOLVENT PHASE gas gas liquid liquid solid solid EXAMPLE air soda pop antifreeze filling seawater brass

Factors Affecting Solubility • • 1. Nature of Solute / Solvent 2. Temperature Increase i) Solid/Liquid ii) gas 3. Pressure Factor i) Solids/Liquids - Very little ii) gas iii) squeezes gas into solution.

Like Dissolves Like • Non-polar in Non-polar • Butter in Oil • Non-polar in polar • Oil in H 2 O • Polar in Polar • C 2 H 5 OH in H 2 O • Ionic compounds in polar solvents • Na. Cl in H 2 O

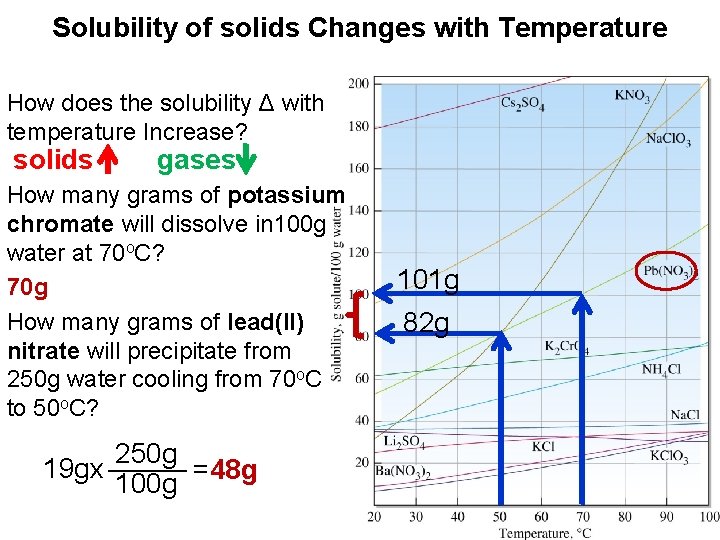
Solubility of solids Changes with Temperature How does the solubility Δ with temperature Increase? solids gases How many grams of potassium chromate will dissolve in 100 g water at 70 o. C? 70 g How many grams of lead(II) nitrate will precipitate from 250 g water cooling from 70 o. C to 50 o. C? 250 g _____ 19 gx =48 g 100 g 101 g 82 g

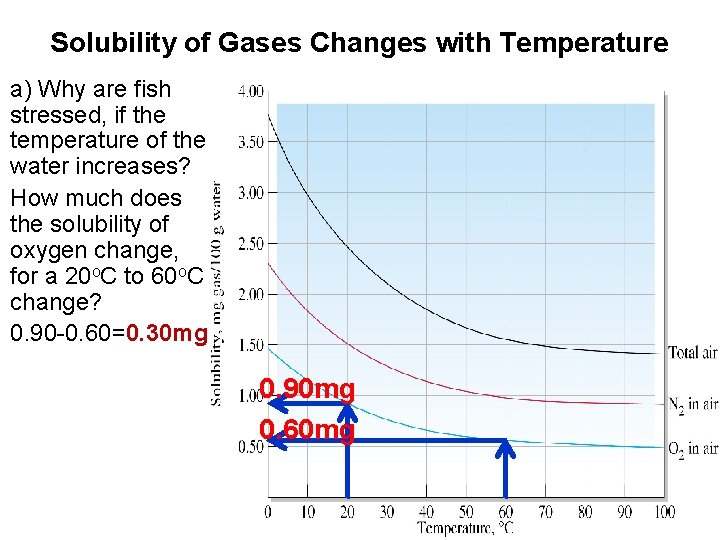
Solubility of Gases Changes with Temperature a) Why are fish stressed, if the temperature of the water increases? How much does the solubility of oxygen change, for a 20 o. C to 60 o. C change? 0. 90 -0. 60=0. 30 mg 0. 90 mg 0. 60 mg

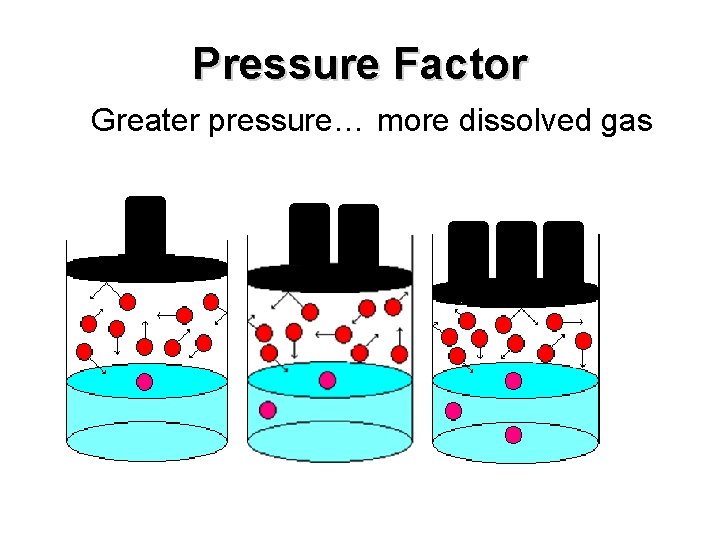
Pressure Factor Greater pressure… more dissolved gas

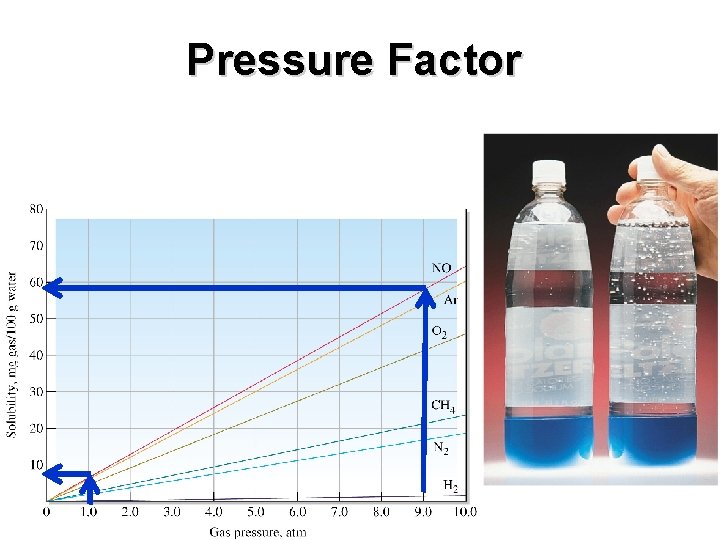
Pressure Factor

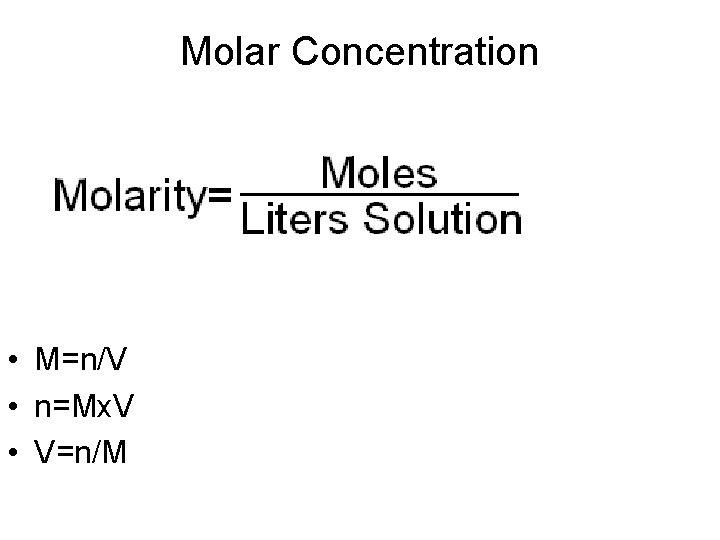
Molar Concentration • M=n/V • n=Mx. V • V=n/M

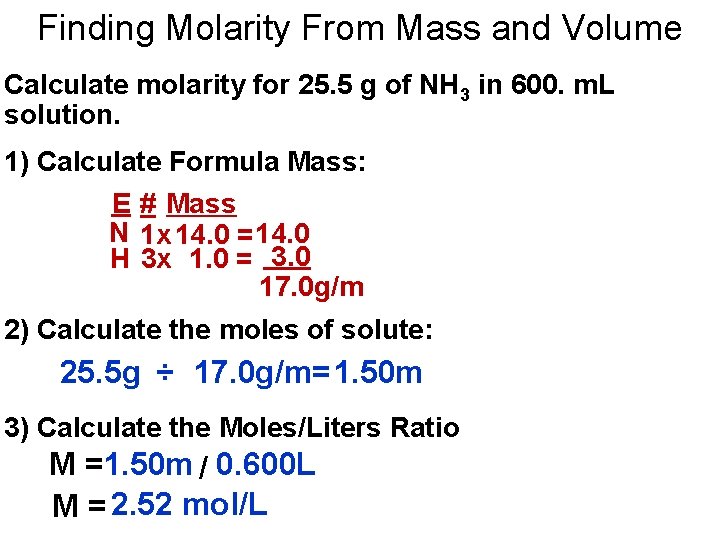
Finding Molarity From Mass and Volume Calculate molarity for 25. 5 g of NH 3 in 600. m. L solution. 1) Calculate Formula Mass: E # Mass N 1 x 14. 0 = 14. 0 H 3 x 1. 0 = 3. 0 17. 0 g/m 2) Calculate the moles of solute: 25. 5 g ÷ 17. 0 g/m=1. 50 m 3) Calculate the Moles/Liters Ratio M =1. 50 m / 0. 600 L M = 2. 52 mol/L

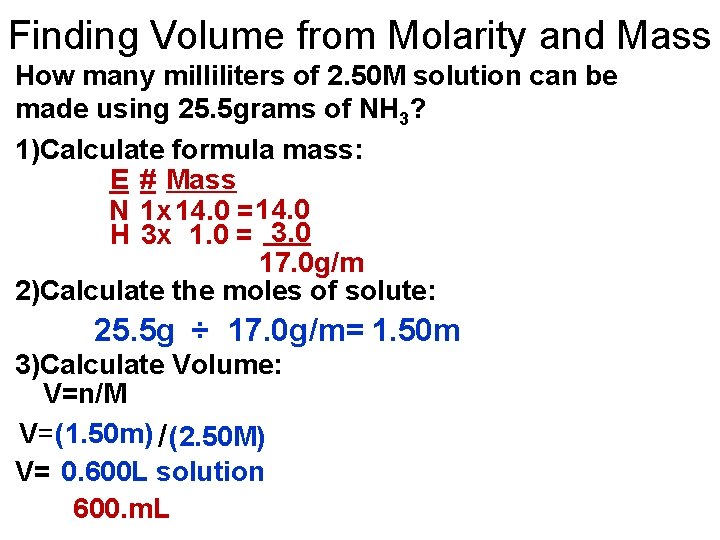
Finding Volume from Molarity and Mass How many milliliters of 2. 50 M solution can be made using 25. 5 grams of NH 3? 1)Calculate formula mass: E # Mass N 1 x 14. 0 = 14. 0 H 3 x 1. 0 = 3. 0 17. 0 g/m 2)Calculate the moles of solute: 25. 5 g ÷ 17. 0 g/m= 1. 50 m 3)Calculate Volume: V=n/M V=(1. 50 m) / (2. 50 M) V= 0. 600 L solution 600. m. L

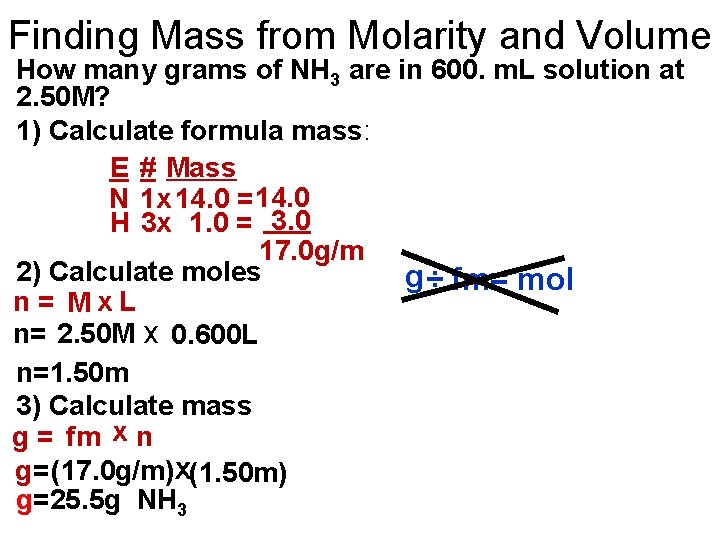
Finding Mass from Molarity and Volume How many grams of NH 3 are in 600. m. L solution at 2. 50 M? 1) Calculate formula mass: E # Mass N 1 x 14. 0 = 14. 0 H 3 x 1. 0 = 3. 0 17. 0 g/m 2) Calculate moles g ÷ fm= mol n= Mx. L n= 2. 50 M x 0. 600 L n=1. 50 m 3) Calculate mass g = fm x n g=(17. 0 g/m)x(1. 50 m) g=25. 5 g NH 3

Notes Two Unit Six– Chapter 13 Solutions • • • Saturated versus Unsaturated Pages 487 -501 Colligative properties of water Forming a Saturated Solution How Does a Solution Form? Colligative Properties Vapor Pressure Boiling and Freezing Point BP Elevation and Freezing FP Depression Calculating Freezing Point Depression Mass

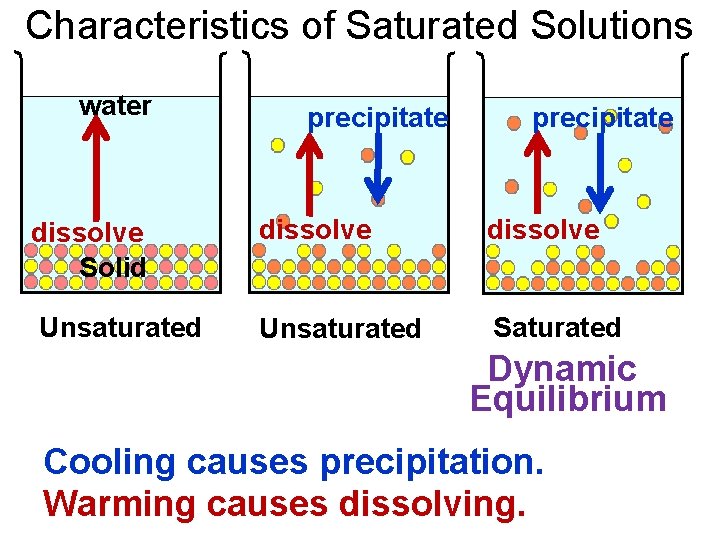
Characteristics of Saturated Solutions water precipitate dissolve Solid dissolve Unsaturated Saturated Dynamic Equilibrium Cooling causes precipitation. Warming causes dissolving.

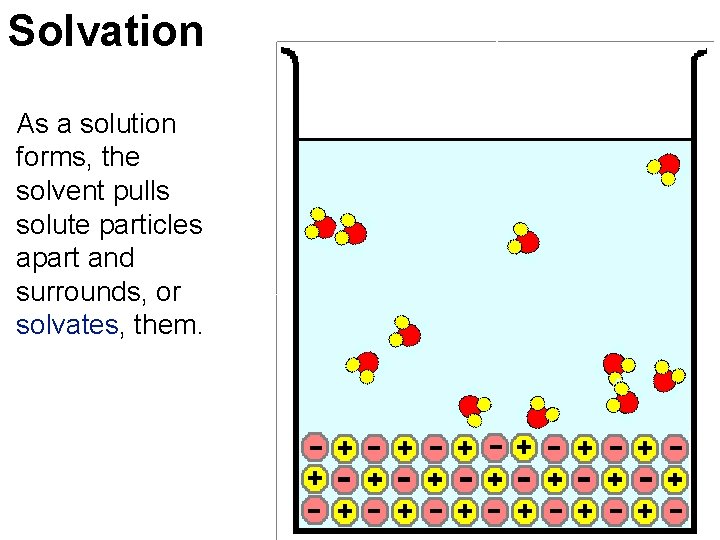
Solvation As a solution forms, the solvent pulls solute particles apart and surrounds, or solvates, them.

Colligative Properties Colligative properties depend on moles dissolved particles. ØVapor pressure lowering ØBoiling point elevation ØMelting point depression ØOsmotic pressure

How do you get from this…

…to this?

Add an ionic compound!

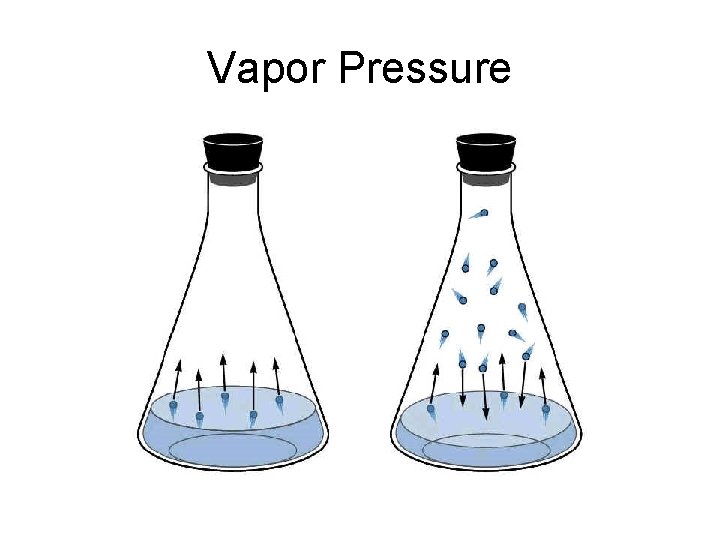
Vapor Pressure

Vapor Pressure Lowering • The particles of solute are surrounded by and attracted to particles of solvent. • Now the solvent particles have less kinetic energy and tend less to escape into the space above the liquid. • So the vapor pressure is less.

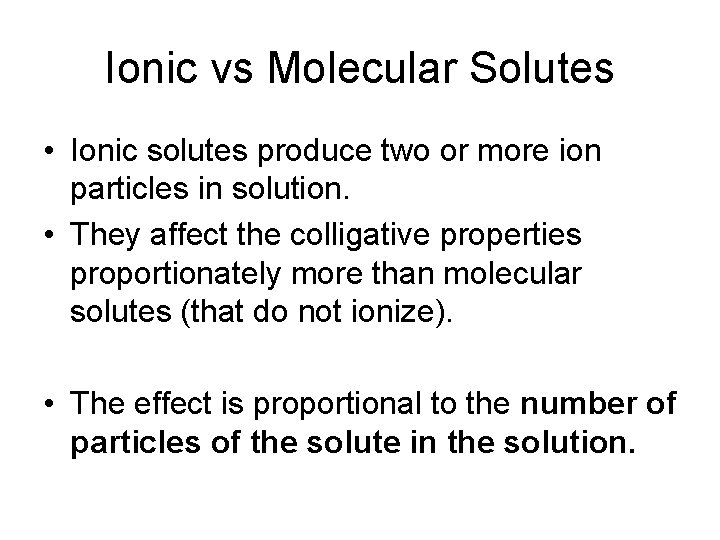
Ionic vs Molecular Solutes • Ionic solutes produce two or more ion particles in solution. • They affect the colligative properties proportionately more than molecular solutes (that do not ionize). • The effect is proportional to the number of particles of the solute in the solution.
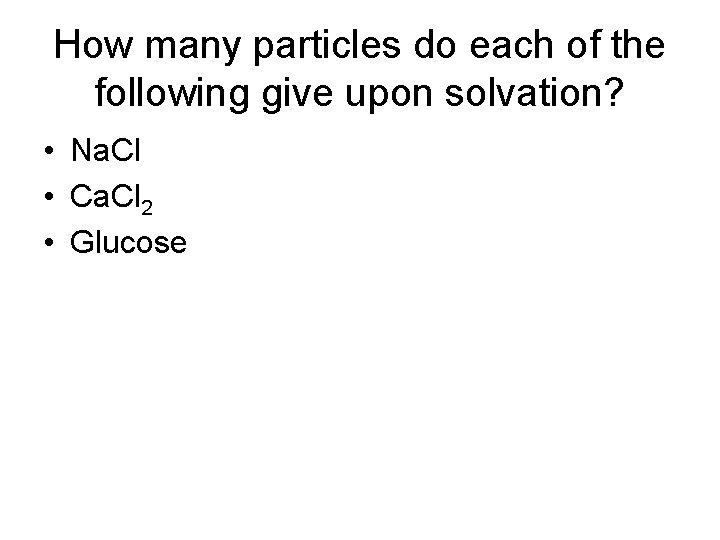
How many particles do each of the following give upon solvation? • Na. Cl • Ca. Cl 2 • Glucose

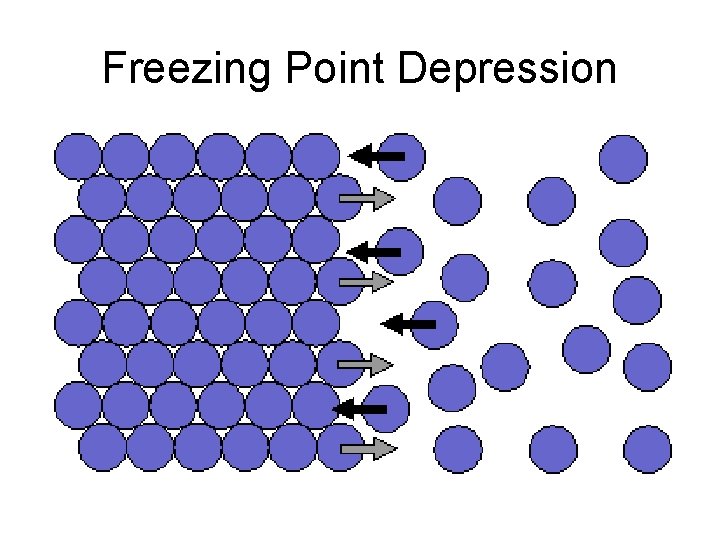
Freezing Point Depression

Example • Salt is added to melt ice by reducing the freezing point of water.

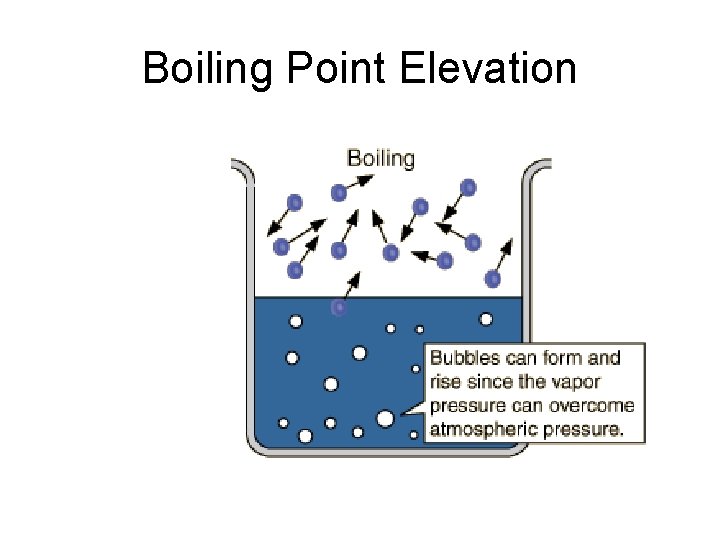
Boiling Point Elevation

Example • Addition of ethylene glycol C 2 H 6 O 2 (antifreeze) to car radiators.

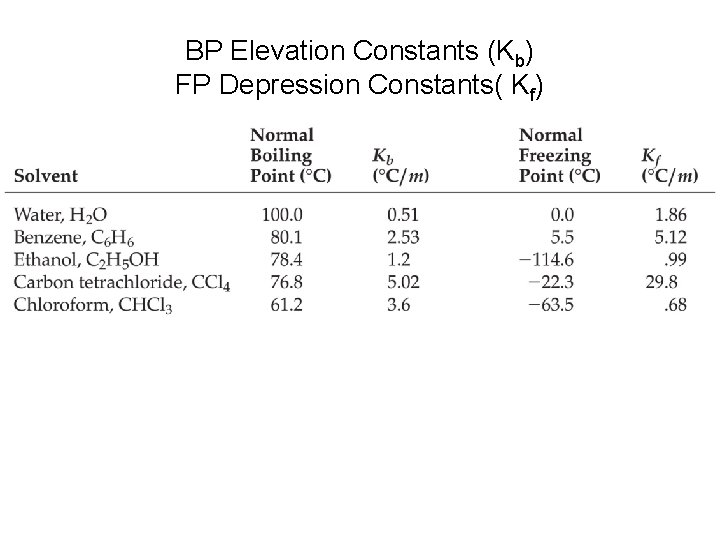
Freezing Point Depression and Boiling Point Elevation • ∆Tb =imkb (for water kb=0. 51 o. C/m) • Freezing Point Depression • ∆Tf=imkf (for water kf=1. 86 o. C/m) • Note: m is the molality of the particles, so if the solute is ionic, multiply by the #of particles it dissociates to. (i is # of particles called van hoff factor)

BP Elevation Constants (Kb) FP Depression Constants( Kf)
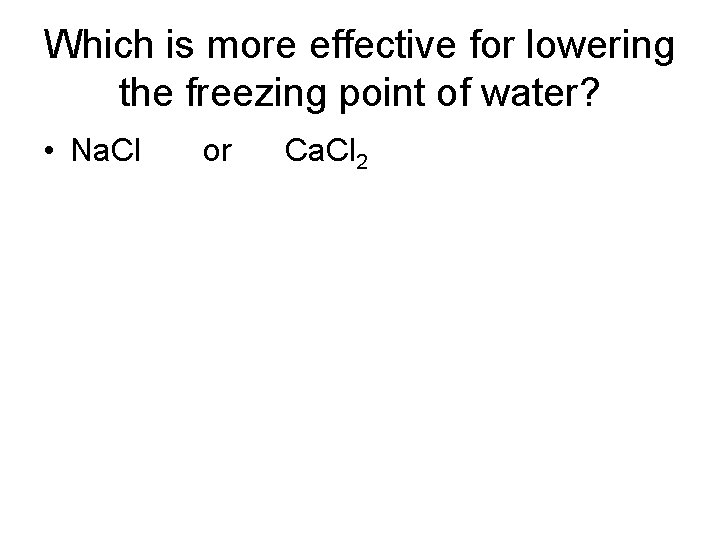
Which is more effective for lowering the freezing point of water? • Na. Cl or Ca. Cl 2

Example 1: • Find the new freezing point of 3 m Na. Cl in water.

Example 2: • Find the new boiling point of 3 m Na. Cl in water.

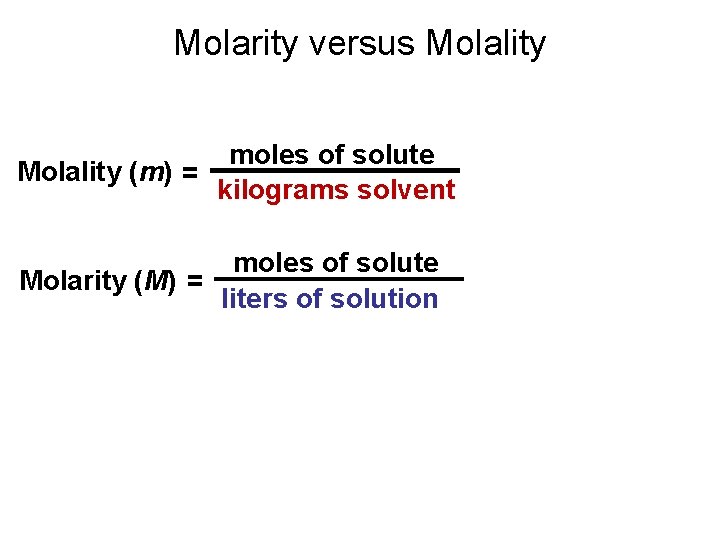
Molarity versus Molality moles of solute ________ Molality (m) = kilograms solvent moles of solute ________ Molarity (M) = liters of solution

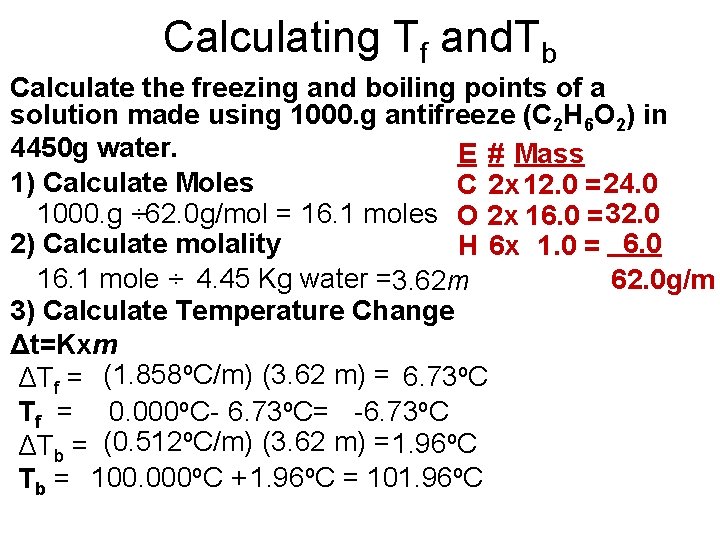
Calculating Tf and. Tb Calculate the freezing and boiling points of a solution made using 1000. g antifreeze (C 2 H 6 O 2) in 4450 g water. E # Mass 1) Calculate Moles C 2 x 12. 0 = 24. 0 1000. g ÷ 62. 0 g/mol = 16. 1 moles O 2 x 16. 0 = 32. 0 2) Calculate molality H 6 x 1. 0 = 6. 0 16. 1 mole ÷ 4. 45 Kg water =3. 62 m 62. 0 g/m 3) Calculate Temperature Change Δt=Kxm ΔTf = (1. 858 o. C/m) (3. 62 m) = 6. 73 o. C Tf = 0. 000 o. C- 6. 73 o. C= -6. 73 o. C ΔTb = (0. 512 o. C/m) (3. 62 m) = 1. 96 o. C Tb = 100. 000 o. C + 1. 96 o. C = 101. 96 o. C

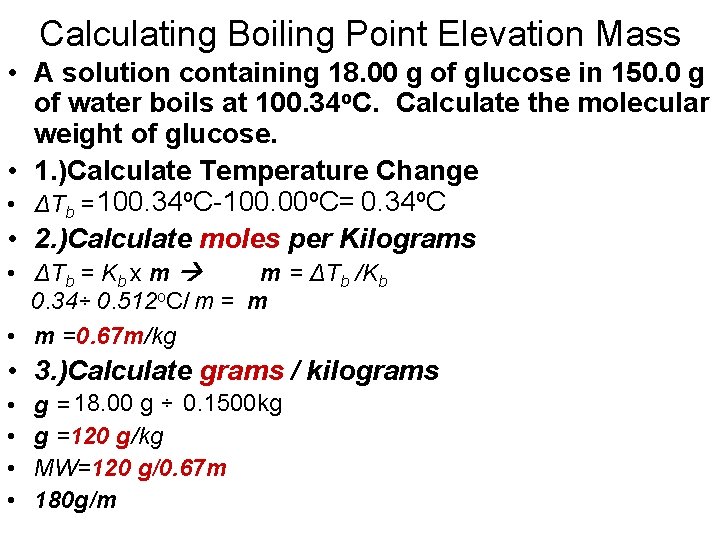
Calculating Boiling Point Elevation Mass • A solution containing 18. 00 g of glucose in 150. 0 g of water boils at 100. 34 o. C. Calculate the molecular weight of glucose. • 1. )Calculate Temperature Change • ΔTb = 100. 34 o. C-100. 00 o. C= 0. 34 o. C • 2. )Calculate moles per Kilograms • ΔTb = Kb x m m = ΔTb /Kb 0. 34÷ 0. 512 o. C/ m = m • m =0. 67 m/kg • 3. )Calculate grams / kilograms • • g = 18. 00 g ÷ 0. 1500 kg g =120 g/kg MW=120 g/0. 67 m 180 g/m

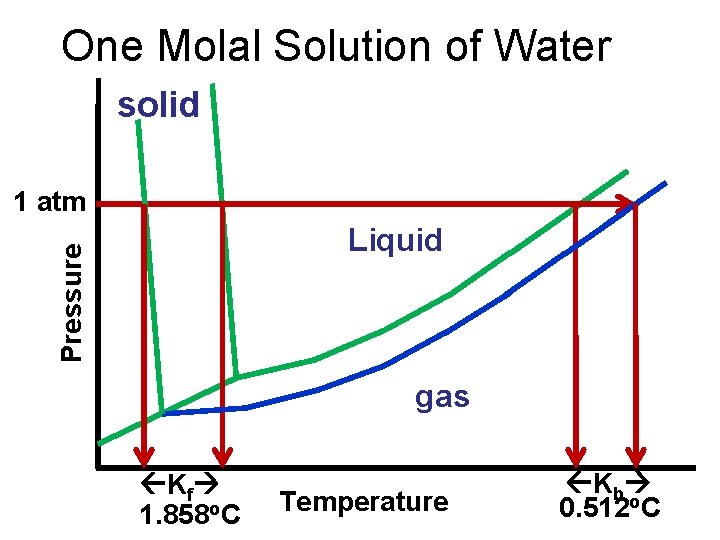
One Molal Solution of Water solid 1 atm Pressure Liquid gas Kf 1. 858 o. C Temperature Kb 0. 512 o. C

Notes Three Unit Six • • • Ice-cream Lab A Calculating Freezing Point Pages 487 -501 Depression Mass Colligative Properties of Electrolytes Distillation Osmotic Pressure Dialysis

Ice-cream

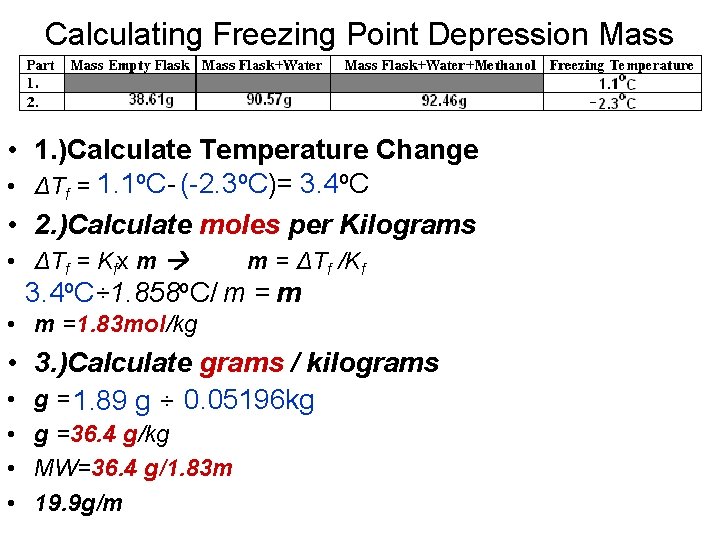
Calculating Freezing Point Depression Mass • 1. )Calculate Temperature Change • ΔTf = 1. 1 o. C- (-2. 3 o. C)= 3. 4 o. C • 2. )Calculate moles per Kilograms • ΔTf = Kfx m m = ΔTf /Kf 3. 4 o. C÷ 1. 858 o. C/ m = m • m =1. 83 mol/kg • 3. )Calculate grams / kilograms • g = 1. 89 g ÷ 0. 05196 kg • g =36. 4 g/kg • MW=36. 4 g/1. 83 m • 19. 9 g/m

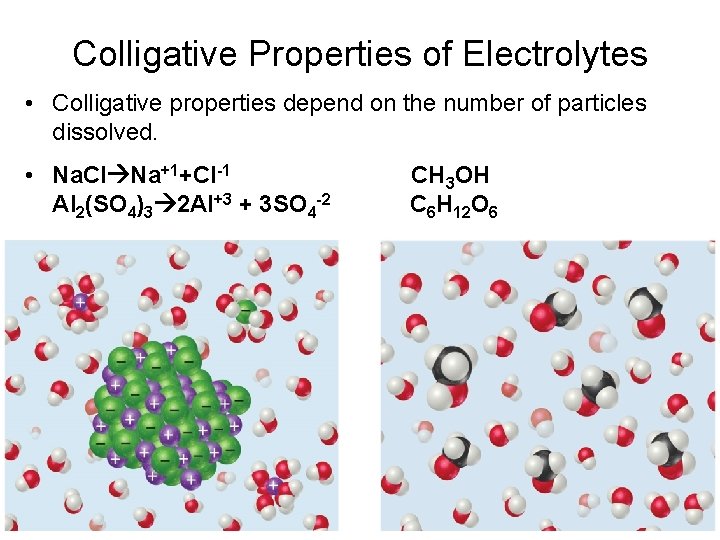
Colligative Properties of Electrolytes • Colligative properties depend on the number of particles dissolved. • Na. Cl Na+1+Cl-1 Al 2(SO 4)3 2 Al+3 + 3 SO 4 -2 CH 3 OH C 6 H 12 O 6

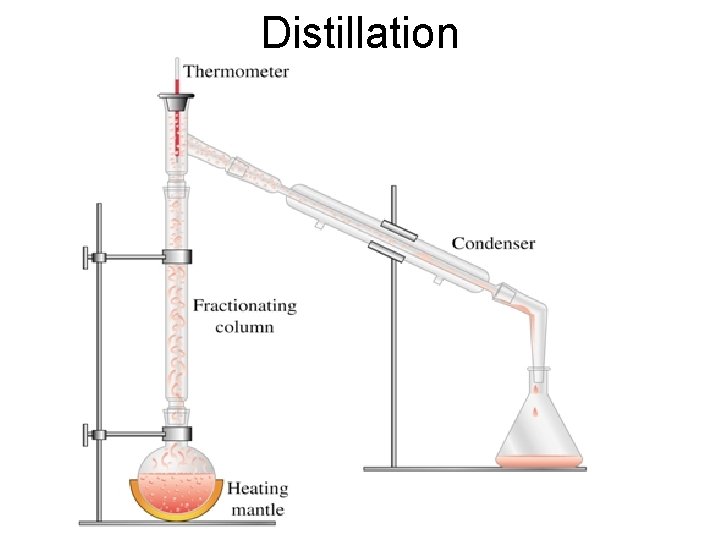
Distillation

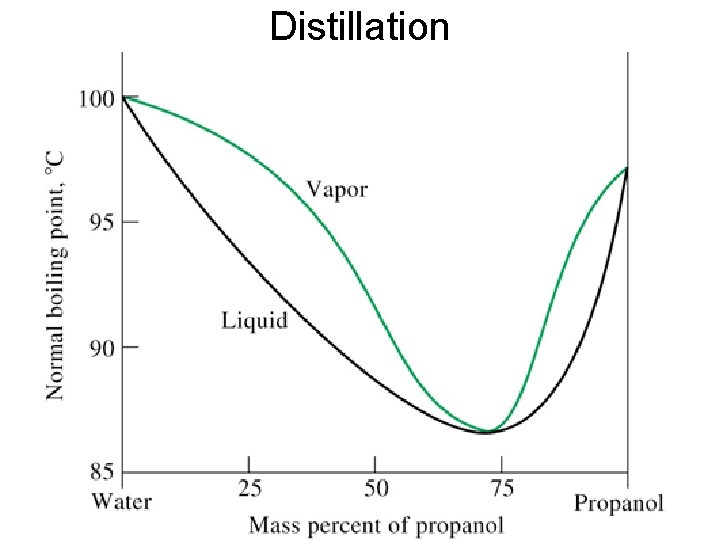
Distillation

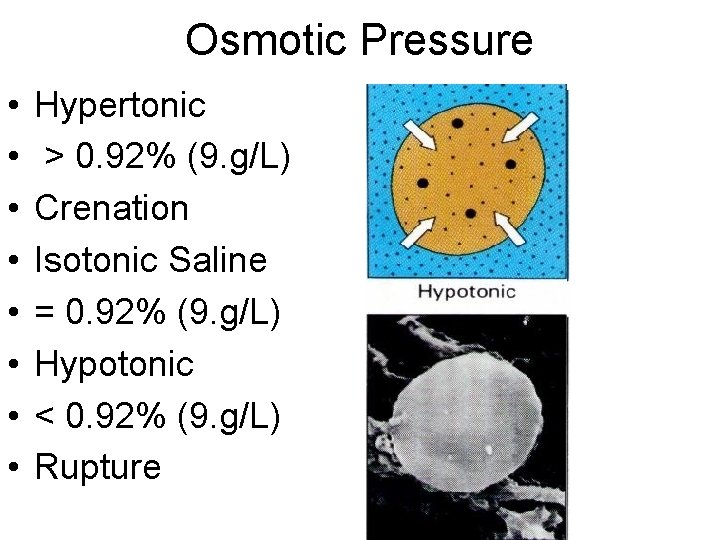
Osmotic Pressure • • Hypertonic > 0. 92% (9. g/L) Crenation Isotonic Saline = 0. 92% (9. g/L) Hypotonic < 0. 92% (9. g/L) Rupture

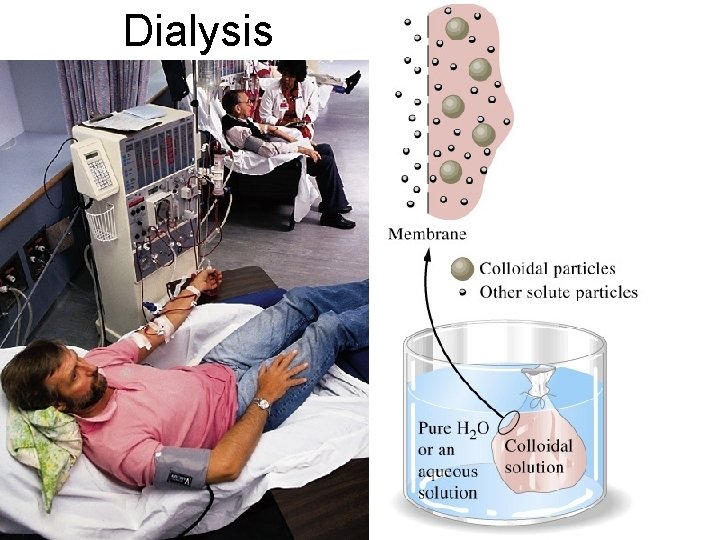
Dialysis

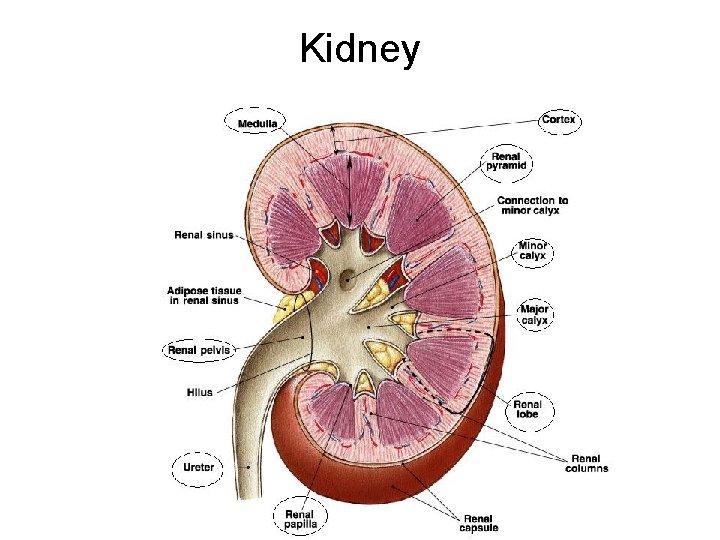
Kidney

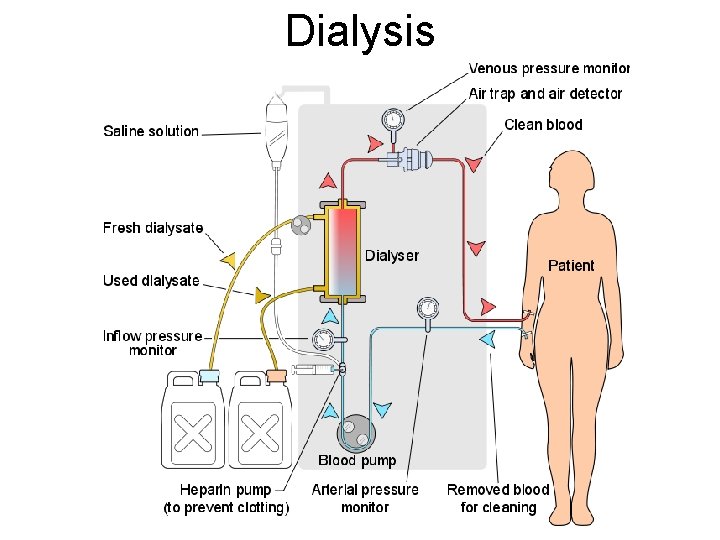
Dialysis

Final Quiz Notes

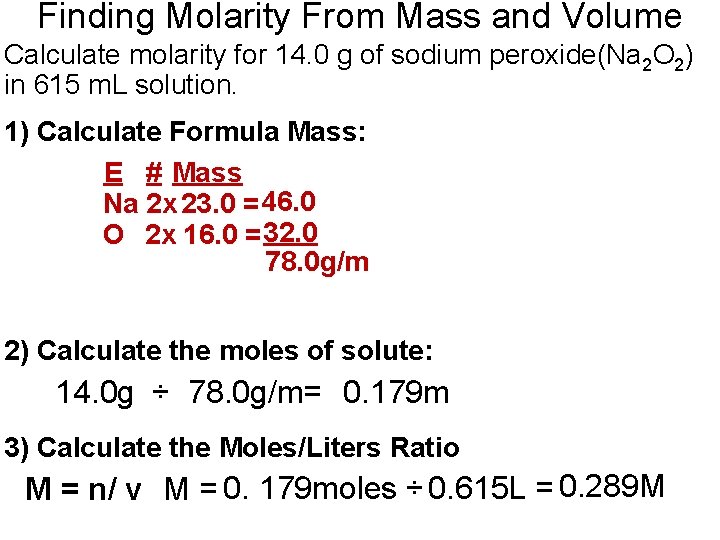
Finding Molarity From Mass and Volume Calculate molarity for 14. 0 g of sodium peroxide(Na 2 O 2) in 615 m. L solution. 1) Calculate Formula Mass: E # Mass Na 2 x 23. 0 = 46. 0 O 2 x 16. 0 = 32. 0 78. 0 g/m 2) Calculate the moles of solute: 14. 0 g ÷ 78. 0 g/m= 0. 179 m 3) Calculate the Moles/Liters Ratio M = n/ v M = 0. 179 moles ÷ 0. 615 L = 0. 289 M

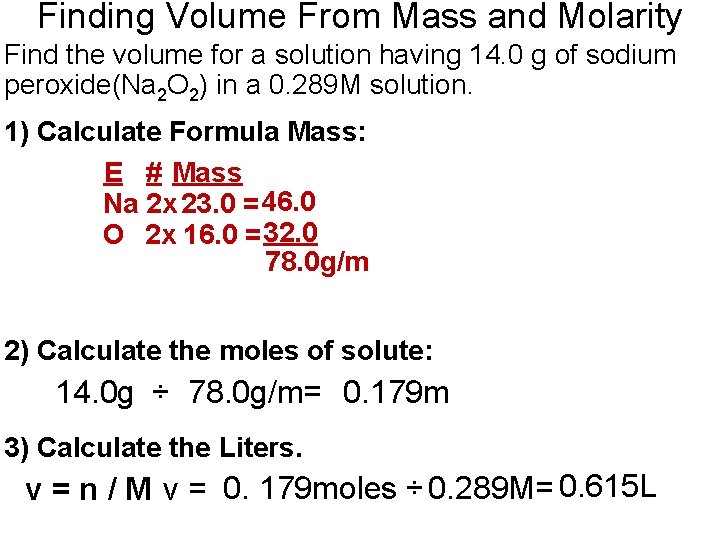
Finding Volume From Mass and Molarity Find the volume for a solution having 14. 0 g of sodium peroxide(Na 2 O 2) in a 0. 289 M solution. 1) Calculate Formula Mass: E # Mass Na 2 x 23. 0 = 46. 0 O 2 x 16. 0 = 32. 0 78. 0 g/m 2) Calculate the moles of solute: 14. 0 g ÷ 78. 0 g/m= 0. 179 m 3) Calculate the Liters. v = n / M v = 0. 179 moles ÷ 0. 289 M= 0. 615 L

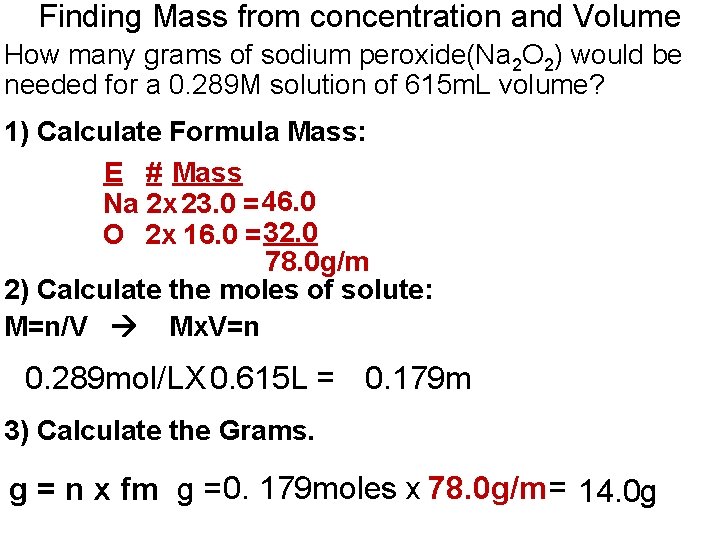
Finding Mass from concentration and Volume How many grams of sodium peroxide(Na 2 O 2) would be needed for a 0. 289 M solution of 615 m. L volume? 1) Calculate Formula Mass: E # Mass Na 2 x 23. 0 = 46. 0 O 2 x 16. 0 = 32. 0 78. 0 g/m 2) Calculate the moles of solute: M=n/V Mx. V=n 0. 289 mol/LX 0. 615 L = 0. 179 m 3) Calculate the Grams. g = n x fm g = 0. 179 moles x 78. 0 g/m= 14. 0 g

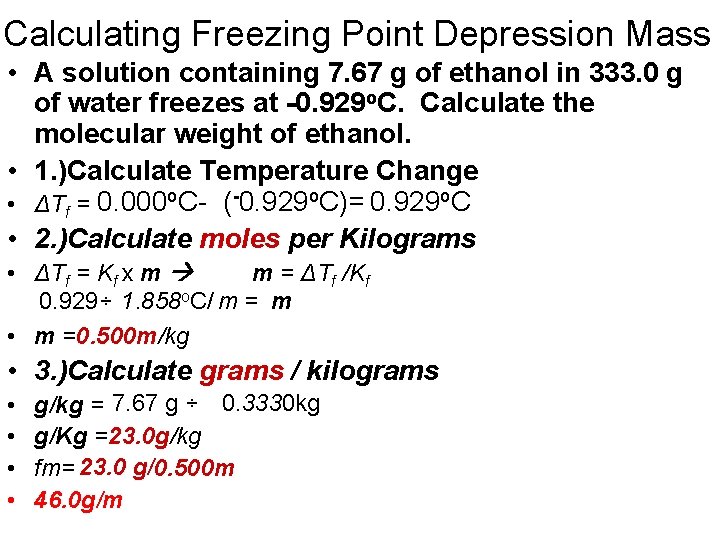
Calculating Freezing Point Depression Mass • A solution containing 7. 67 g of ethanol in 333. 0 g of water freezes at -0. 929 o. C. Calculate the molecular weight of ethanol. • 1. )Calculate Temperature Change • ΔTf = 0. 000 o. C- (-0. 929 o. C)= 0. 929 o. C • 2. )Calculate moles per Kilograms • ΔTf = Kf x m m = ΔTf /Kf 0. 929÷ 1. 858 o. C/ m = m • m =0. 500 m/kg • 3. )Calculate grams / kilograms • • g/kg = 7. 67 g ÷ 0. 3330 kg g/Kg =23. 0 g/kg fm= 23. 0 g/0. 500 m 46. 0 g/m

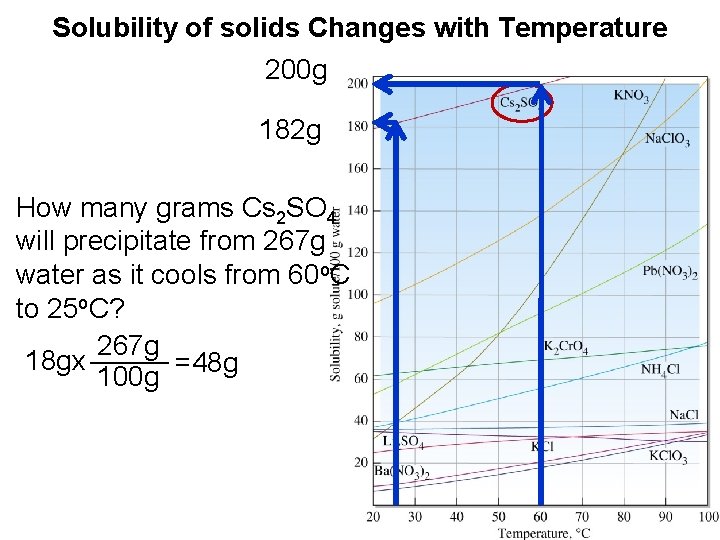
Solubility of solids Changes with Temperature 200 g 182 g How many grams Cs 2 SO 4 will precipitate from 267 g water as it cools from 60 o. C to 25 o. C? 267 g _____ 18 gx =48 g 100 g

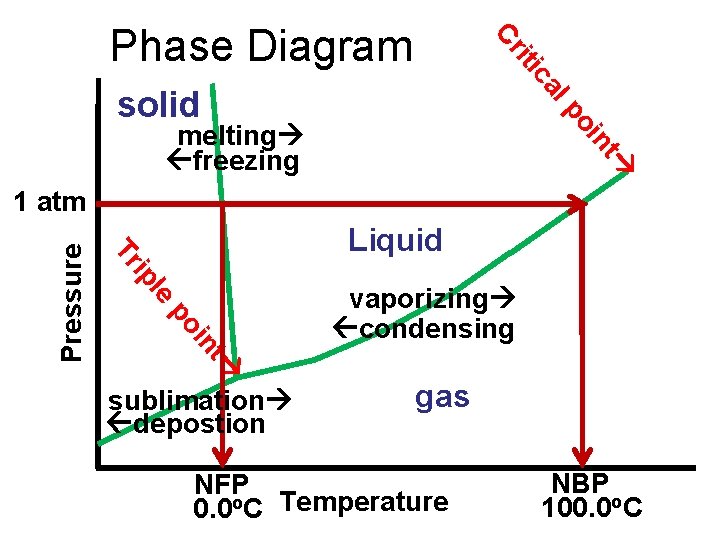
ca iti Cr Phase Diagram lp solid nt oi melting freezing Liquid le ip Tr po t in Pressure 1 atm sublimation depostion vaporizing condensing gas NFP 0. 0 o. C Temperature NBP 100. 0 o. C
end

Mg of gas per 100 grams of water 80 70 60 50 40 30 20 10 0 0 1. 0 2. 0 3. 0 4. 0 5. 0 6. 0 7. 0 8. 0 9. 0 10. 0 Gas pressure in atmospheres

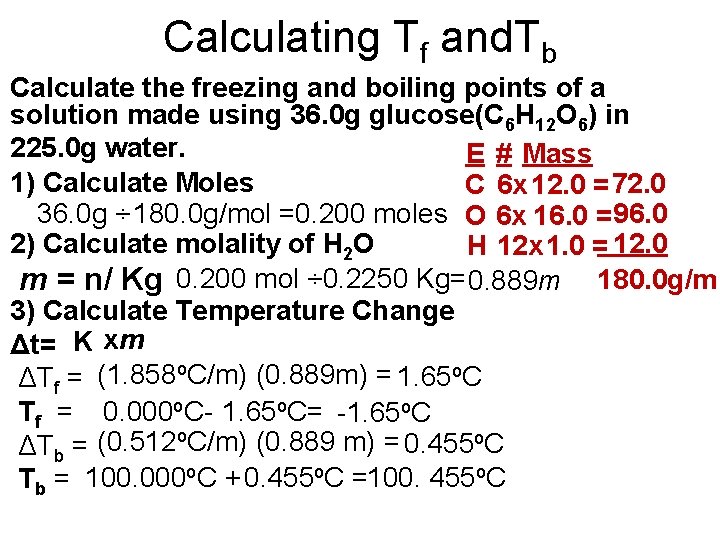
Calculating Tf and. Tb Calculate the freezing and boiling points of a solution made using 36. 0 g glucose(C 6 H 12 O 6) in 225. 0 g water. E # Mass 1) Calculate Moles C 6 x 12. 0 = 72. 0 36. 0 g ÷ 180. 0 g/mol =0. 200 moles O 6 x 16. 0 = 96. 0 2) Calculate molality of H 2 O H 12 x 1. 0 = 12. 0 m = n/ Kg 0. 200 mol ÷ 0. 2250 Kg= 0. 889 m 180. 0 g/m 3) Calculate Temperature Change Δt= K xm ΔTf = (1. 858 o. C/m) (0. 889 m) = 1. 65 o. C Tf = 0. 000 o. C- 1. 65 o. C= -1. 65 o. C ΔTb = (0. 512 o. C/m) (0. 889 m) = 0. 455 o. C Tb = 100. 000 o. C + 0. 455 o. C =100. 455 o. C

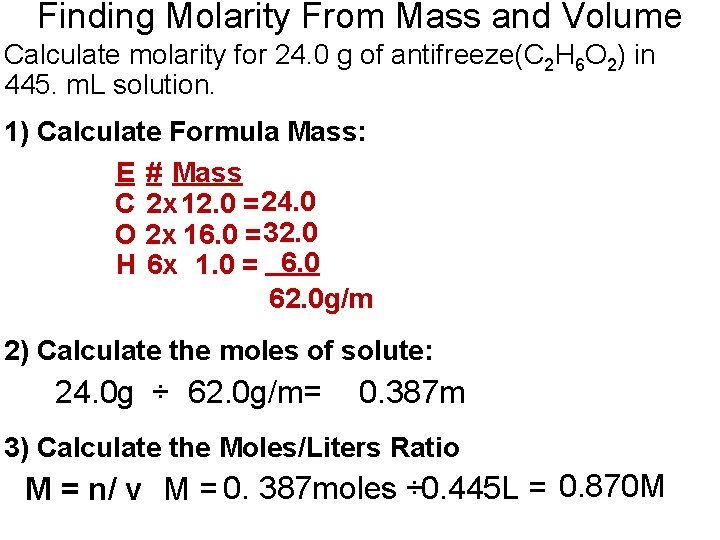
Finding Molarity From Mass and Volume Calculate molarity for 24. 0 g of antifreeze(C 2 H 6 O 2) in 445. m. L solution. 1) Calculate Formula Mass: E # Mass C 2 x 12. 0 = 24. 0 O 2 x 16. 0 = 32. 0 H 6 x 1. 0 = 6. 0 62. 0 g/m 2) Calculate the moles of solute: 24. 0 g ÷ 62. 0 g/m= 0. 387 m 3) Calculate the Moles/Liters Ratio M = n/ v M = 0. 387 moles ÷ 0. 445 L = 0. 870 M

Seven/Eight Rows

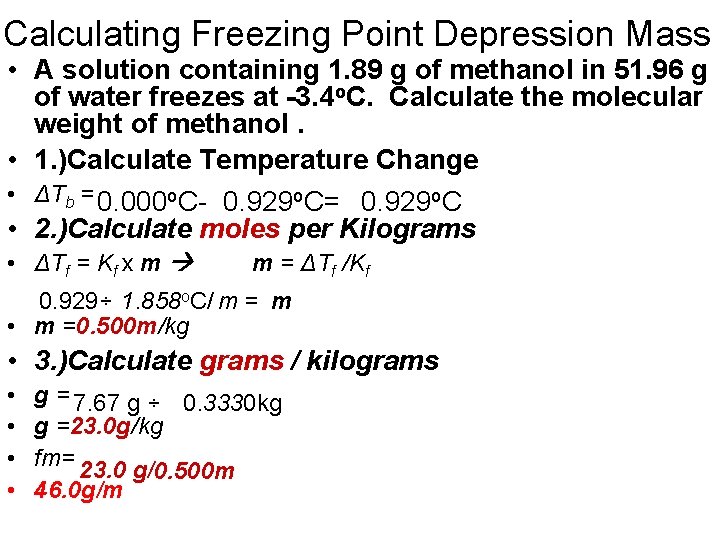
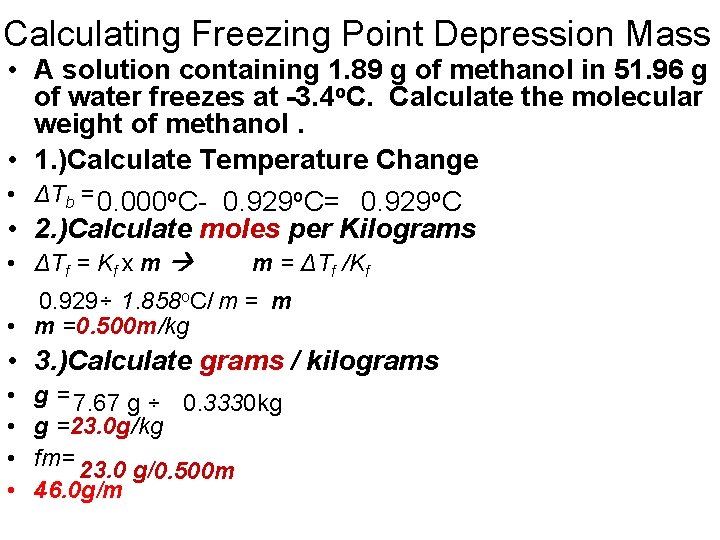
Calculating Freezing Point Depression Mass • A solution containing 1. 89 g of methanol in 51. 96 g of water freezes at -3. 4 o. C. Calculate the molecular weight of methanol. • 1. )Calculate Temperature Change • ΔTb = 0. 000 o. C- 0. 929 o. C= 0. 929 o. C • 2. )Calculate moles per Kilograms • ΔTf = Kf x m m = ΔTf /Kf 0. 929÷ 1. 858 o. C/ m = m • m =0. 500 m/kg • 3. )Calculate grams / kilograms • • g = 7. 67 g ÷ 0. 3330 kg g =23. 0 g/kg fm= 23. 0 g/ 0. 500 m 46. 0 g/m

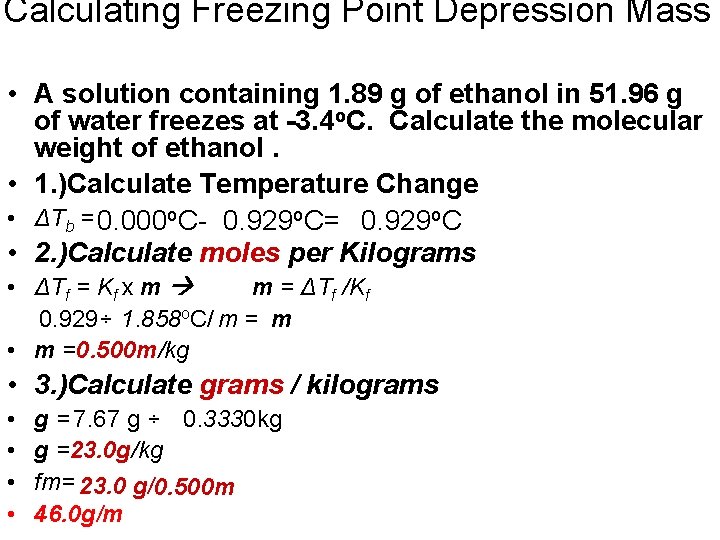
Calculating Freezing Point Depression Mass • A solution containing 1. 89 g of ethanol in 51. 96 g of water freezes at -3. 4 o. C. Calculate the molecular weight of ethanol. • 1. )Calculate Temperature Change • ΔTb = 0. 000 o. C- 0. 929 o. C= 0. 929 o. C • 2. )Calculate moles per Kilograms • ΔTf = Kf x m m = ΔTf /Kf 0. 929÷ 1. 858 o. C/ m = m • m =0. 500 m/kg • 3. )Calculate grams / kilograms • • g = 7. 67 g ÷ 0. 3330 kg g =23. 0 g/kg fm= 23. 0 g/0. 500 m 46. 0 g/m

Calculating Freezing Point Depression Mass • A solution containing 1. 89 g of methanol in 51. 96 g of water freezes at -3. 4 o. C. Calculate the molecular weight of methanol. • 1. )Calculate Temperature Change • ΔTb = 0. 000 o. C- 0. 929 o. C= 0. 929 o. C • 2. )Calculate moles per Kilograms • ΔTf = Kf x m m = ΔTf /Kf 0. 929÷ 1. 858 o. C/ m = m • m =0. 500 m/kg • 3. )Calculate grams / kilograms • • g = 7. 67 g ÷ 0. 3330 kg g =23. 0 g/kg fm= 23. 0 g/ 0. 500 m 46. 0 g/m