Notes One Unit Five Characteristics of Gases Pressure




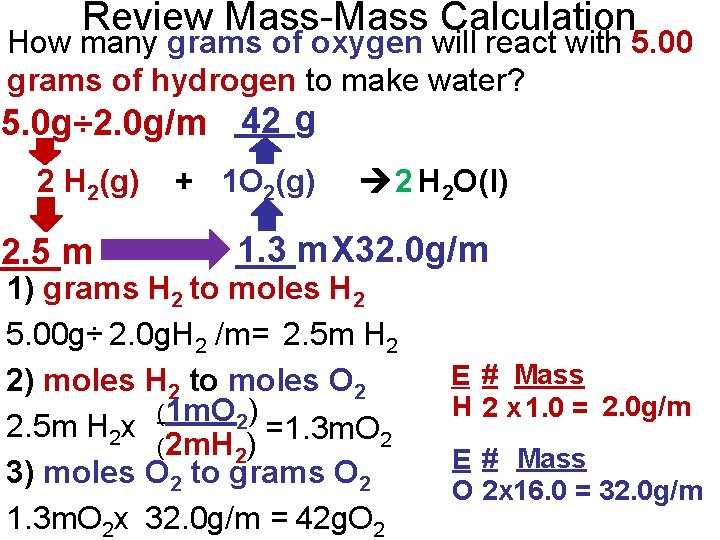

- Slides: 31

Notes One Unit Five Characteristics of Gases Pressure of fluids Standard Temperature and Pressure Converting Pressures Gas Laws Pages 422 -440

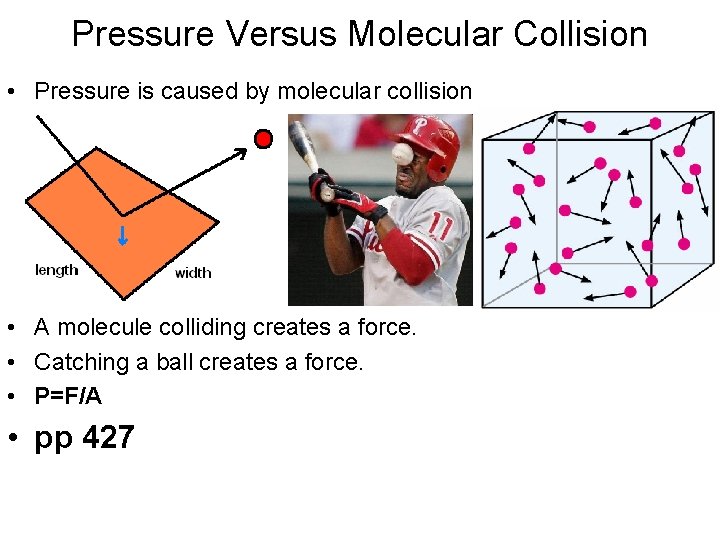
Pressure Versus Molecular Collision • Pressure is caused by molecular collision • A molecule colliding creates a force. • Catching a ball creates a force. • P=F/A • pp 427

Pressure viewed as created in a fluid • Created by the weight • The deeper you go, the more weight. pp 427

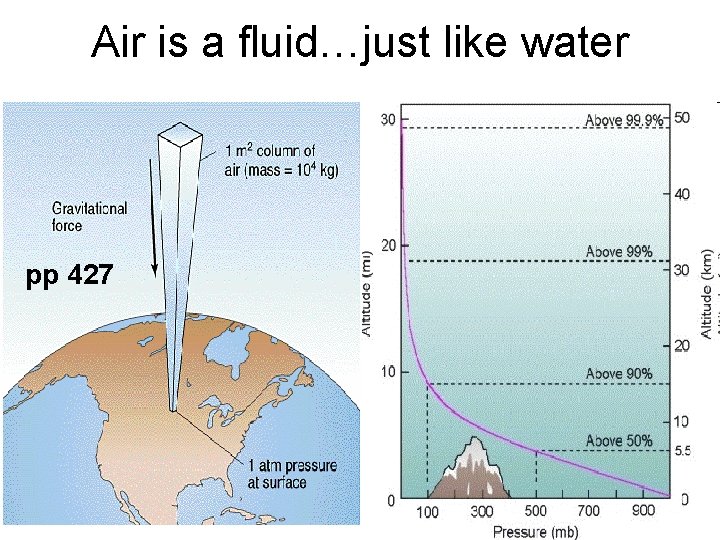
Air is a fluid…just like water pp 427

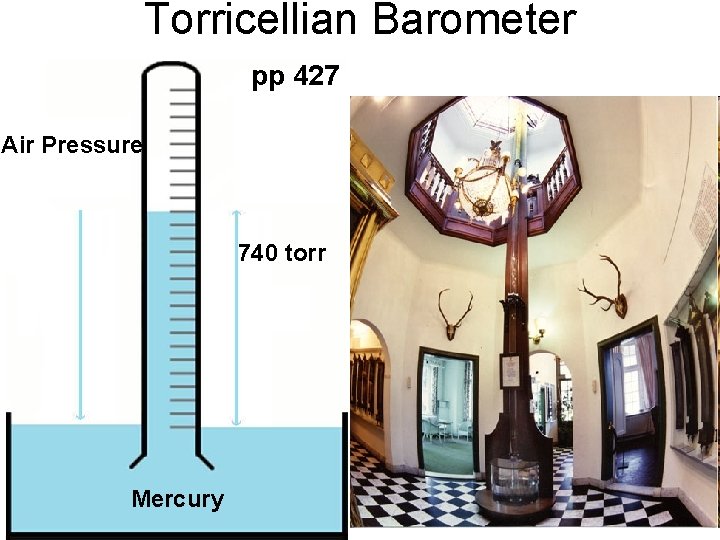
Torricellian Barometer pp 427 Air Pressure 780 760 torr 740 torr Mercury

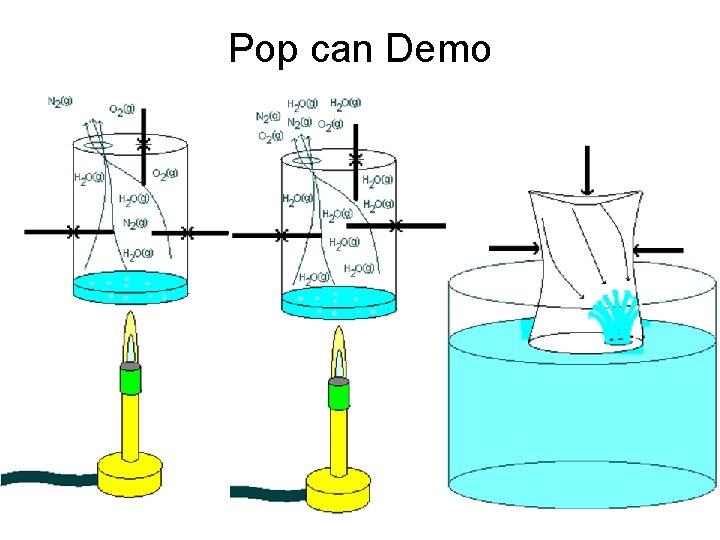
Pop can Demo

Magdeburg plates Demo

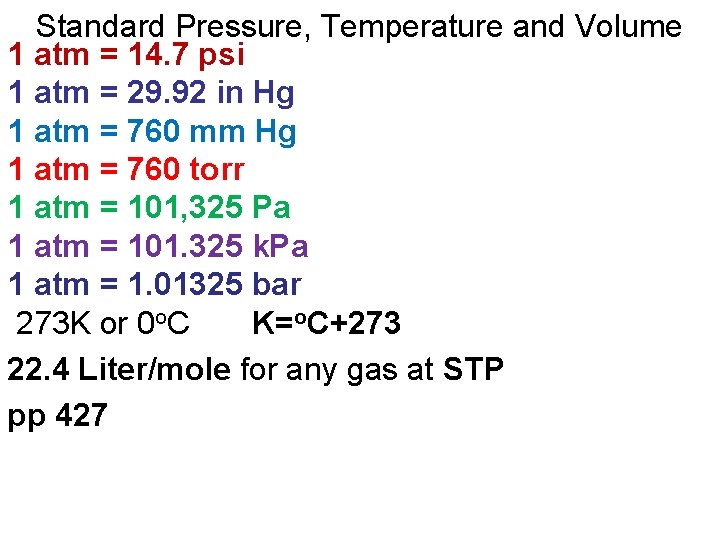
Standard Pressure, Temperature and Volume 1 atm = 14. 7 psi 1 atm = 29. 92 in Hg 1 atm = 760 mm Hg 1 atm = 760 torr 1 atm = 101, 325 Pa 1 atm = 101. 325 k. Pa 1 atm = 1. 01325 bar 273 K or 0 o. C K=o. C+273 22. 4 Liter/mole for any gas at STP pp 427

Converting Pressures • Convert 25 lb/in 2 to torr • Convert 75 Kpa to in Hg

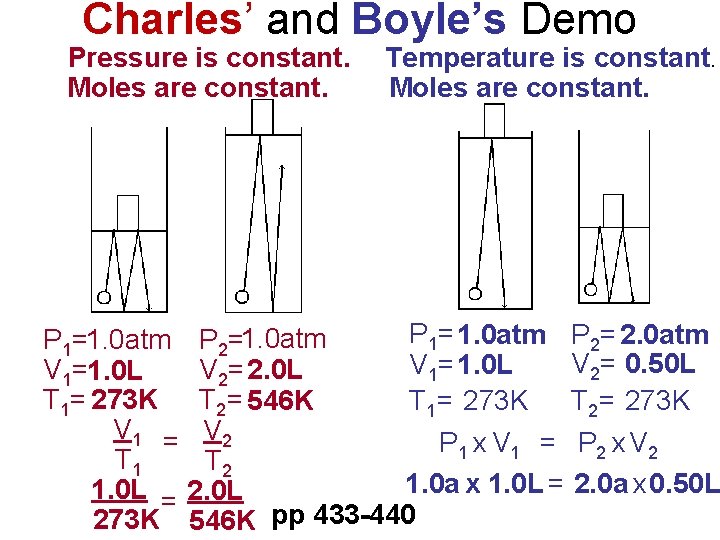
Charles’ and Boyle’s Demo Pressure is constant. Moles are constant. Temperature is constant. Moles are constant. P 1= 1. 0 atm P 2= 2. 0 atm P 1=1. 0 atm P 2=1. 0 atm V 2= 0. 50 L V 1=1. 0 L V 2= 2. 0 L T 1= 273 K T 2= 546 K T 1= 273 K T 2= 273 K V 1 = V 2 P 1 x V 1 = P 2 x V 2 T 1 T 2 1. 0 a x 1. 0 L = 2. 0 a x 0. 50 L 1. 0 L = 2. 0 L 273 K 546 K pp 433 -440

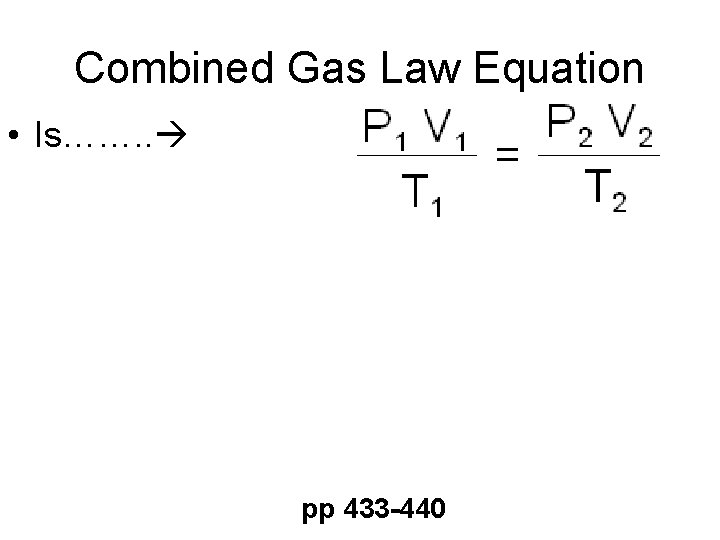
Combined Gas Law Equation • Is……. . pp 433 -440

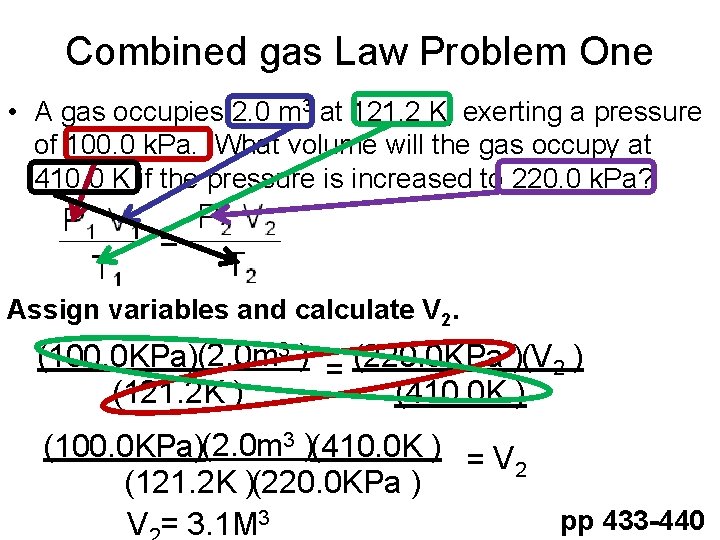
Combined gas Law Problem One • A gas occupies 2. 0 m 3 at 121. 2 K, exerting a pressure of 100. 0 k. Pa. What volume will the gas occupy at 410. 0 K if the pressure is increased to 220. 0 k. Pa? Assign variables and calculate V 2. (100. 0 KPa)(2. 0 m 3 ) = (220. 0 KPa )(V 2 ) (121. 2 K ) (410. 0 K ) (100. 0 KPa)(2. 0 m 3 )(410. 0 K ) = V 2 (121. 2 K )(220. 0 KPa ) V = 3. 1 M 3 pp 433 -440

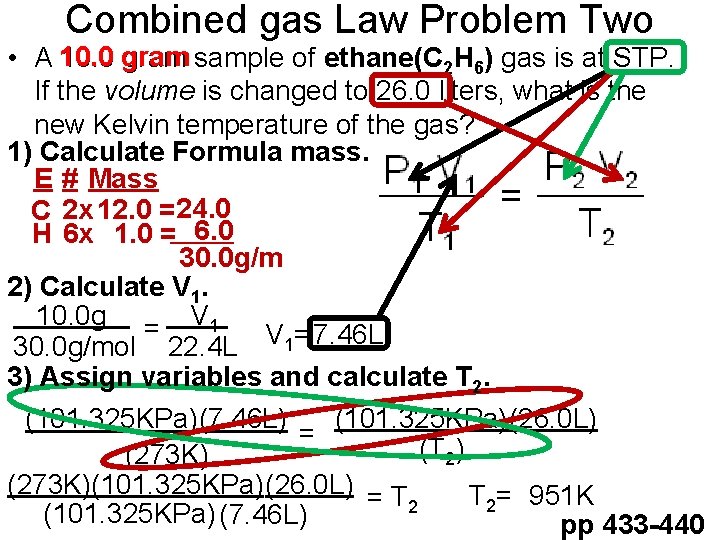
Combined gas Law Problem Two • A 10. 0 gram sample of ethane(C 2 H 6) gas is at STP. If the volume is changed to 26. 0 liters, what is the new Kelvin temperature of the gas? 1) Calculate Formula mass. E # Mass C 2 x 12. 0 = 24. 0 H 6 x 1. 0 = 6. 0 30. 0 g/m 2) Calculate V 1. 10. 0 g = V 1 30. 0 g/mol 22. 4 L V 1=7. 46 L 3) Assign variables and calculate T 2. (101. 325 KPa)(26. 0 L) (101. 325 KPa)(7. 46 L) = (T 2) (273 K)(101. 325 KPa)(26. 0 L) = T T 2= 951 K 2 (101. 325 KPa) (7. 46 L) pp 433 -440

Notes Two Unit Five Grahams’ Law Calculation Review Mass-Mass Calculation Mass-Volume Calculation @STP Volume-Mass Calculation @STP Pages 441 -450

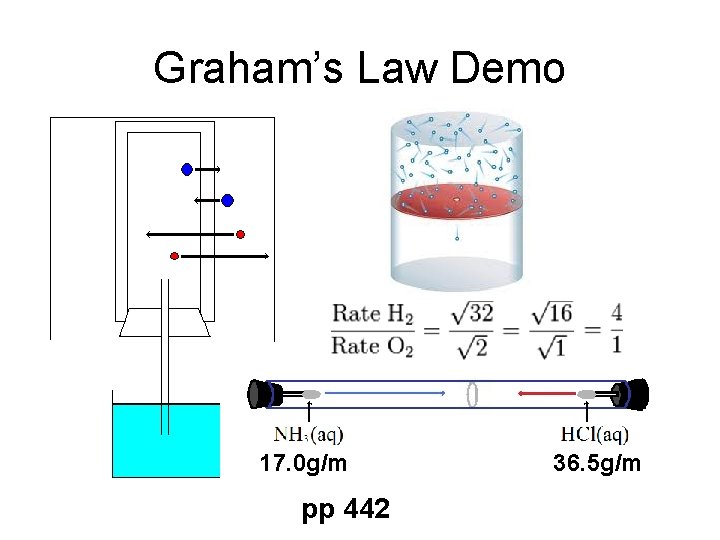
Graham’s Law Demo 17. 0 g/m pp 442 36. 5 g/m

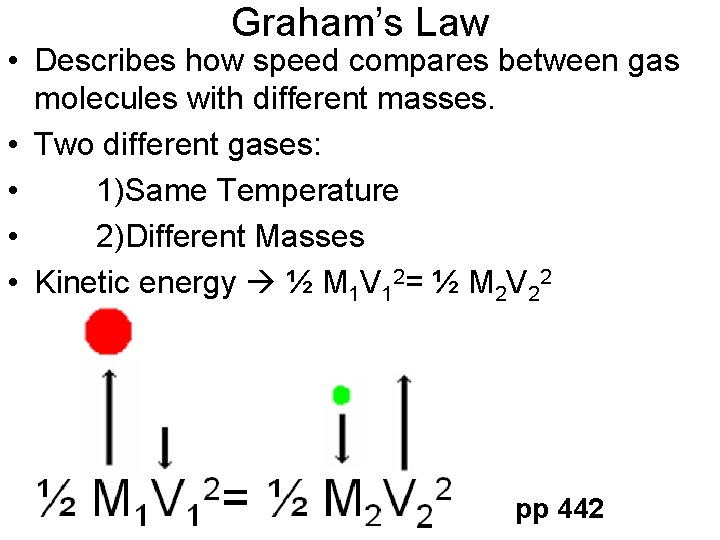
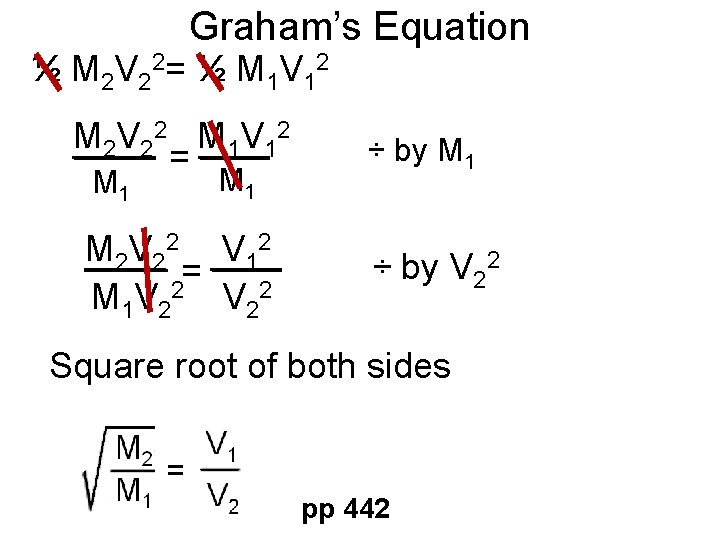
Graham’s Law • Describes how speed compares between gas molecules with different masses. • Two different gases: • 1)Same Temperature • 2)Different Masses • Kinetic energy ½ M 1 V 12= ½ M 2 V 22 pp 442

Graham’s Equation ½ M 2 V 22= ½ M 1 V 12 M 2 V 2 2 M 1 V 1 2 = M 1 M 2 V 22 V 122 V = 12 2 M 1 V 2 ÷ by M 1 ÷ by V 22 Square root of both sides pp 442

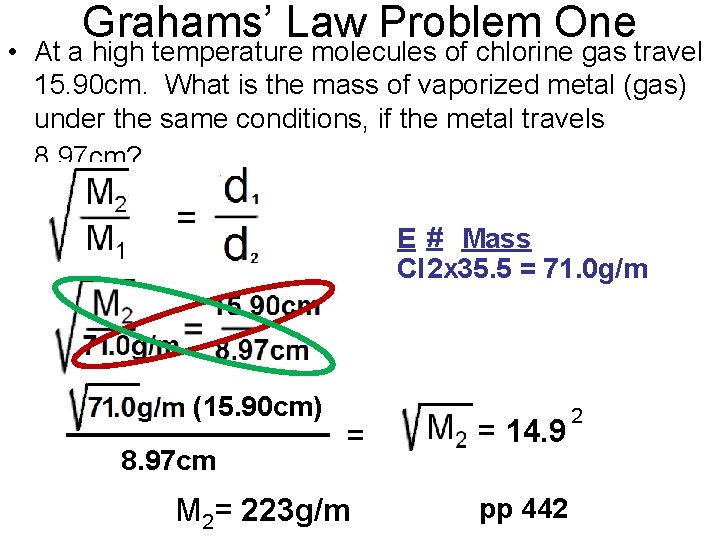
Grahams’ Law Problem One • At a high temperature molecules of chlorine gas travel 15. 90 cm. What is the mass of vaporized metal (gas) under the same conditions, if the metal travels 8. 97 cm? E # Mass Cl 2 x 35. 5 = 71. 0 g/m (15. 90 cm) 8. 97 cm = M 2= 223 g/m = 14. 9 pp 442 2

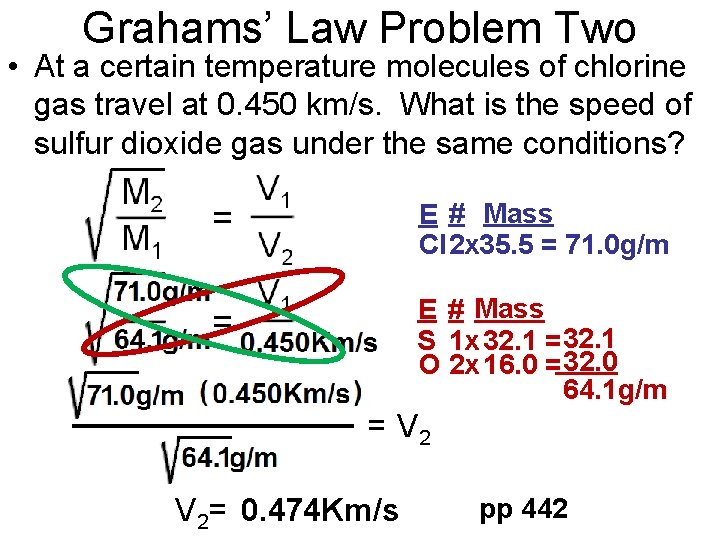
Grahams’ Law Problem Two • At a certain temperature molecules of chlorine gas travel at 0. 450 km/s. What is the speed of sulfur dioxide gas under the same conditions? E # Mass Cl 2 x 35. 5 = 71. 0 g/m E # Mass S 1 x 32. 1 = 32. 1 O 2 x 16. 0 = 32. 0 64. 1 g/m = V 2= 0. 474 Km/s pp 442
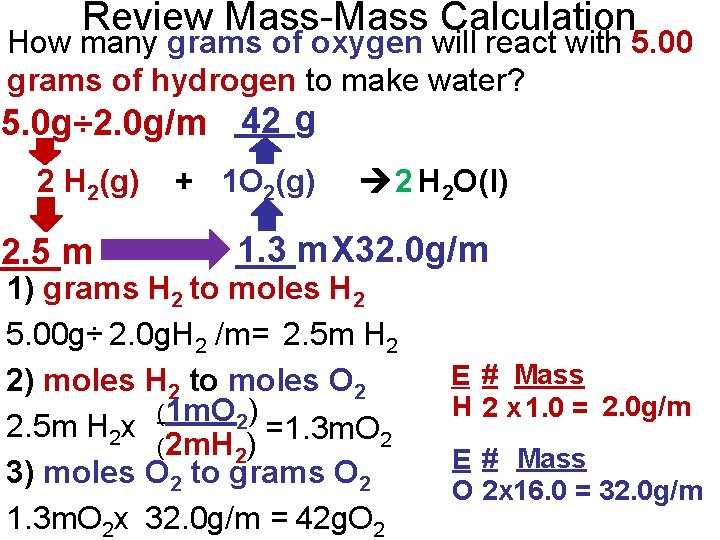
Review Mass-Mass Calculation How many grams of oxygen will react with 5. 00 grams of hydrogen to make water? 42 5. 0 g÷ 2. 0 g/m ___g 2 H 2(g) ___m 2. 5 + 1 O 2(g) 2 H 2 O(l) ___m 1. 3 X 32. 0 g/m 1) grams H 2 to moles H 2 5. 00 g÷ 2. 0 g. H 2 /m= 2. 5 m H 2 2) moles H 2 to moles O 2 (1 m. O 2) 2. 5 m H 2 x =1. 3 m. O 2 (2 m. H 2) 3) moles O 2 to grams O 2 1. 3 m. O 2 x 32. 0 g/m = 42 g. O 2 E # Mass H 2 x 1. 0 = 2. 0 g/m E # Mass O 2 x 16. 0 = 32. 0 g/m

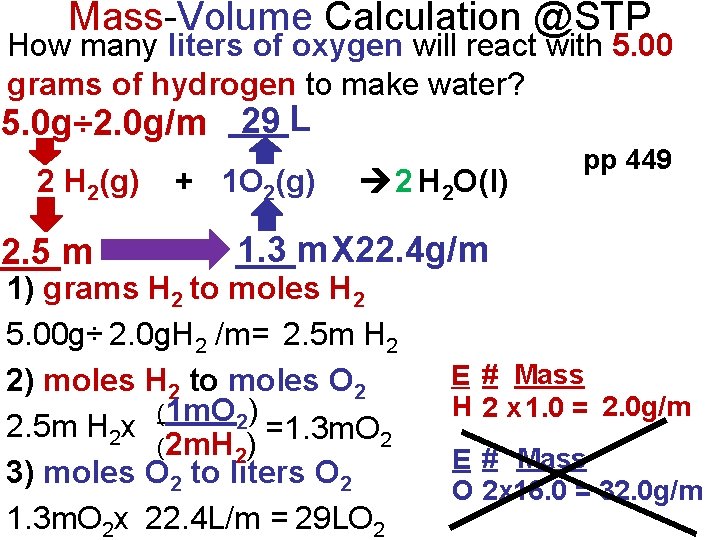
Mass-Volume Calculation @STP How many liters of oxygen will react with 5. 00 grams of hydrogen to make water? 29 5. 0 g÷ 2. 0 g/m ___L 2 H 2(g) ___m 2. 5 + 1 O 2(g) 2 H 2 O(l) pp 449 ___m 1. 3 X 22. 4 g/m 1) grams H 2 to moles H 2 5. 00 g÷ 2. 0 g. H 2 /m= 2. 5 m H 2 2) moles H 2 to moles O 2 (1 m. O 2) 2. 5 m H 2 x =1. 3 m. O 2 (2 m. H 2) 3) moles O 2 to liters O 2 1. 3 m. O 2 x 22. 4 L/m = 29 LO 2 E # Mass H 2 x 1. 0 = 2. 0 g/m E # Mass O 2 x 16. 0 = 32. 0 g/m

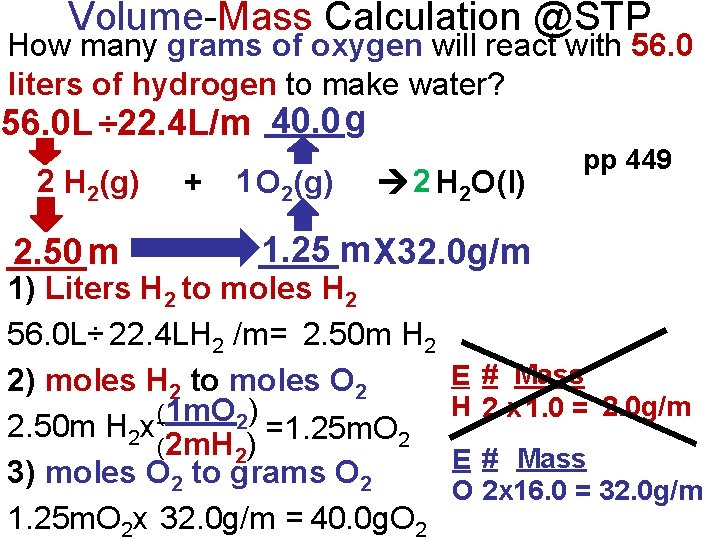
Volume-Mass Calculation @STP How many grams of oxygen will react with 56. 0 liters of hydrogen to make water? 40. 0 56. 0 L ÷ 22. 4 L/m ____g 2 H 2(g) ____m 2. 50 + 1 O 2(g) 2 H 2 O(l) pp 449 ____m 1. 25 X 32. 0 g/m 1) Liters H 2 to moles H 2 56. 0 L÷ 22. 4 LH 2 /m= 2. 50 m H 2 2) moles H 2 to moles O 2 (1 m. O 2) 2. 50 m H 2 x =1. 25 m. O 2 (2 m. H 2) 3) moles O 2 to grams O 2 1. 25 m. O 2 x 32. 0 g/m = 40. 0 g. O 2 E # Mass H 2 x 1. 0 = 2. 0 g/m E # Mass O 2 x 16. 0 = 32. 0 g/m

Notes Three Unit Five • Kinetic theory of gases • Molar volume @ Non-STP Conditions • R is Universal Gas Constant Pages 452 -459

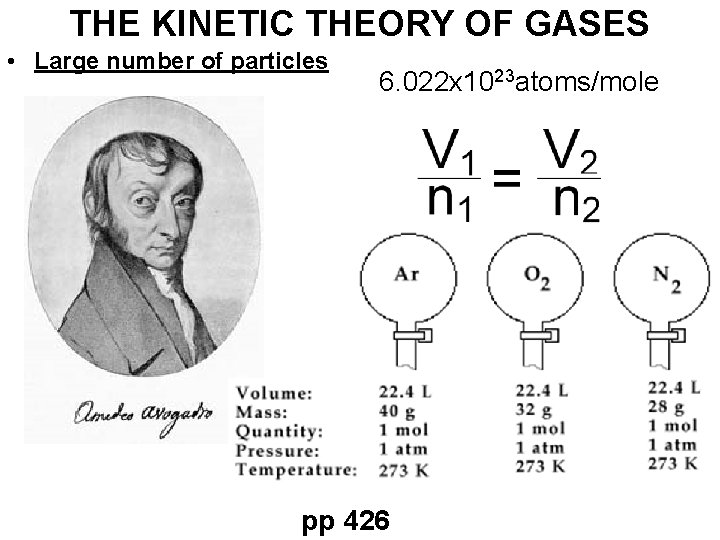
THE KINETIC THEORY OF GASES • Large number of particles 6. 022 x 1023 atoms/mole pp 426

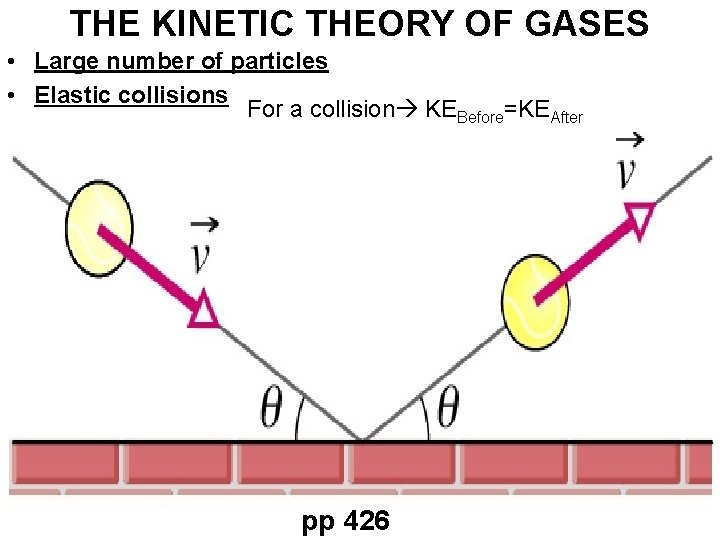
THE KINETIC THEORY OF GASES • Large number of particles • Elastic collisions For a collision KEBefore=KEAfter pp 426

THE KINETIC THEORY OF GASES • Large number of particles • Elastic collisions • No external forces pp 433 -440

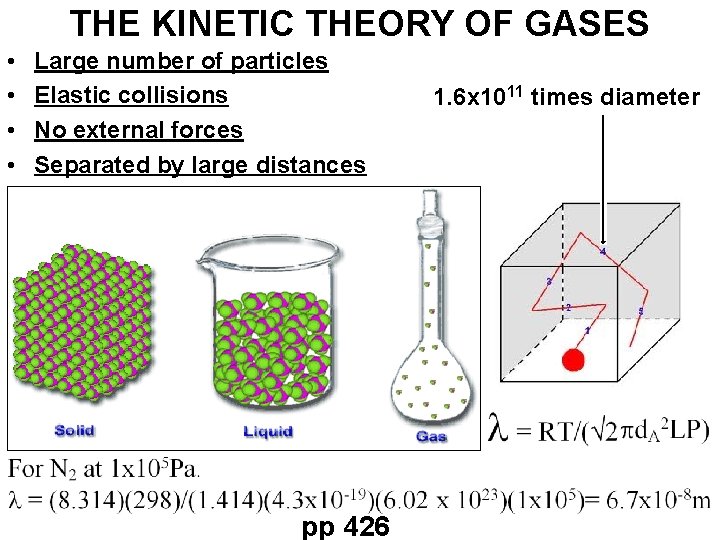
THE KINETIC THEORY OF GASES • • Large number of particles Elastic collisions No external forces Separated by large distances pp 426 1. 6 x 1011 times diameter

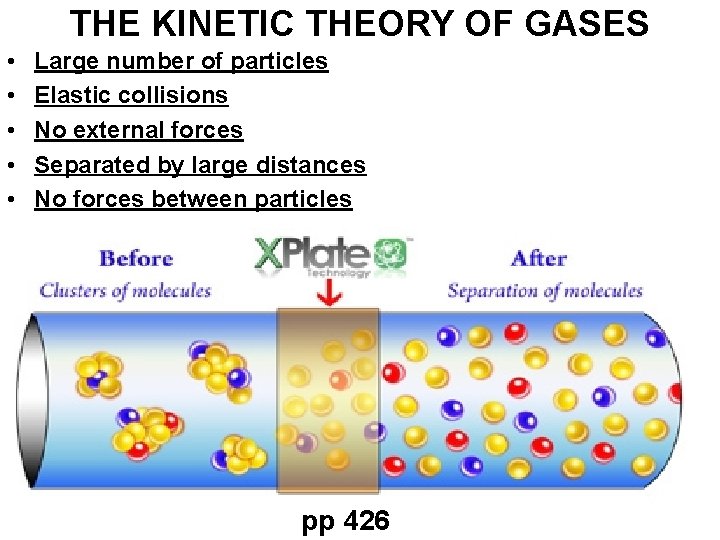
THE KINETIC THEORY OF GASES • • • Large number of particles Elastic collisions No external forces Separated by large distances No forces between particles pp 426

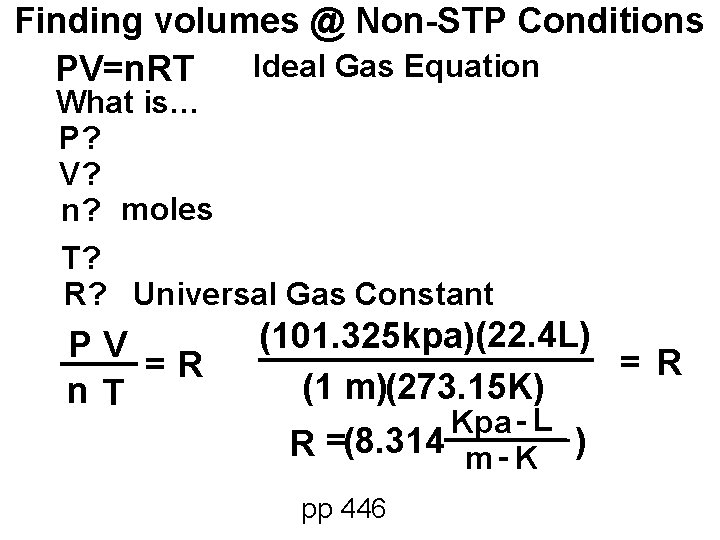
Finding volumes @ Non-STP Conditions Ideal Gas Equation PV=n. RT What is… P? V? n? moles T? R? Universal Gas Constant PV =R n. T (101. 325 kpa)(22. 4 L) = R (1 m)(273. 15 K) Kpa - L R =(8. 314 m - K ) pp 446

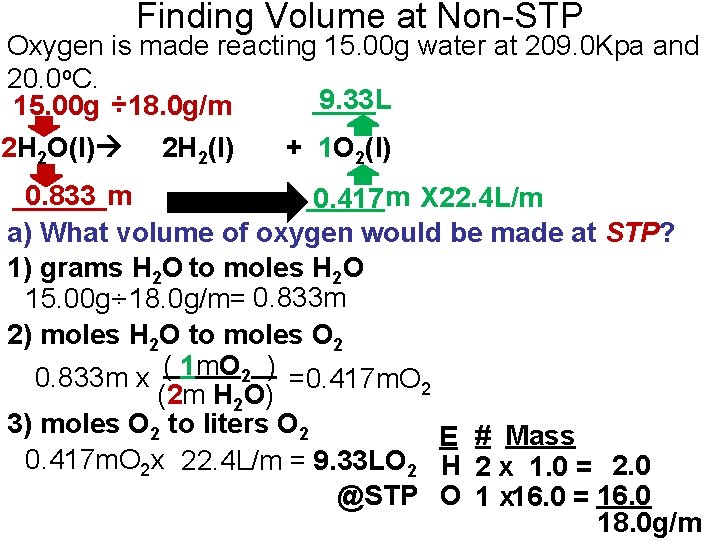
Finding Volume at Non-STP Oxygen is made reacting 15. 00 g water at 209. 0 Kpa and 20. 0 o. C. ____L 9. 33 15. 00 g ÷ 18. 0 g/m 2 H 2 O(l) 2 H 2(l) + 1 O 2(l) ______m 0. 833 _____m 0. 417 X 22. 4 L/m a) What volume of oxygen would be made at STP? 1) grams H 2 O to moles H 2 O 15. 00 g÷ 18. 0 g/m= 0. 833 m 2) moles H 2 O to moles O 2 0. 833 m x ( 1 m. O 2 ) =0. 417 m. O 2 (2 m H 2 O) 3) moles O 2 to liters O 2 E # Mass 0. 417 m. O 2 x 22. 4 L/m = 9. 33 LO 2 H 2 x 1. 0 = 2. 0 @STP O 1 x 16. 0 = 16. 0 18. 0 g/m

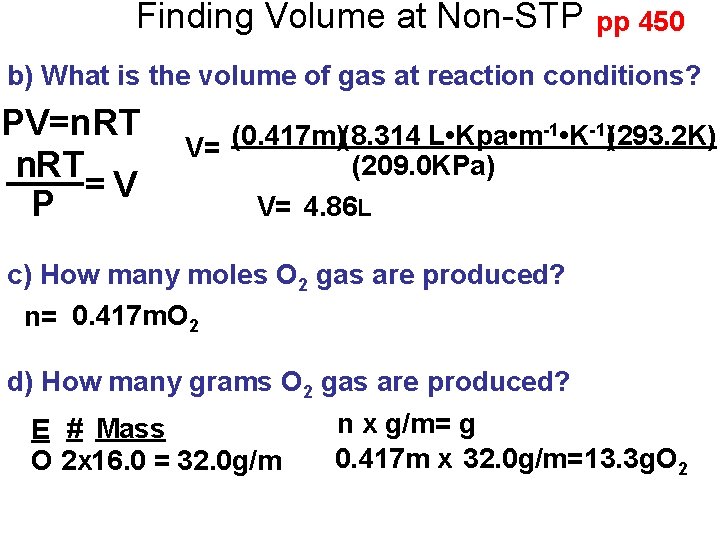
Finding Volume at Non-STP pp 450 b) What is the volume of gas at reaction conditions? PV=n. RT n. R T =V P -1 • K-1)(293. 2 K) (0. 417 m)(8. 314 L • Kpa • m V= (209. 0 KPa) V= 4. 86 L c) How many moles O 2 gas are produced? n= 0. 417 m. O 2 d) How many grams O 2 gas are produced? n x g/m= g E # Mass 0. 417 m x 32. 0 g/m=13. 3 g. O 2 x 16. 0 = 32. 0 g/m