Notes on the Periodic Table Antoine Lavoisier v
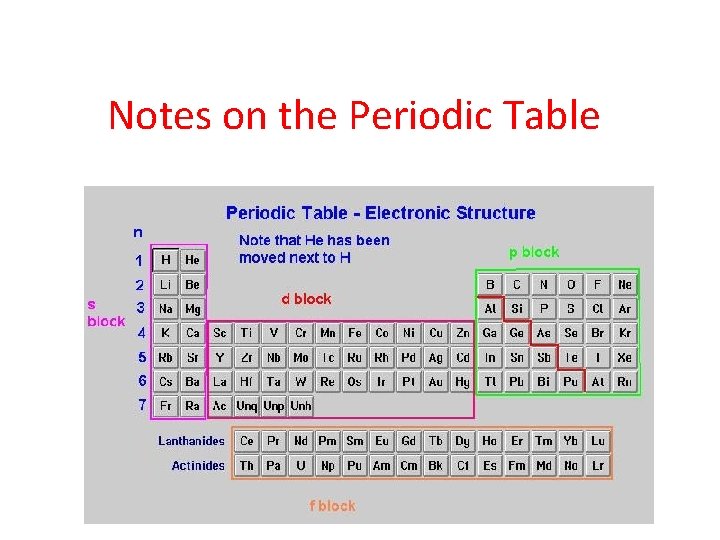
Notes on the Periodic Table

Antoine Lavoisier v 1790’s • made a list of all the known elements; there were 23 v By the 1870’s, there were 70 known elements v A system of organization was needed

Dimitri Medeleev https: //encryptedtbn 1. gstatic. com/images? q=t bn: ANd 9 Gc. Tze. KUu. TTXAu. Y 9 m tgz. BOe. JXppeol. Cc 5 l. Ou. LAVqtg. ZBIZOu. NKQA • Was given credit for the first periodic table even though John Newland also developed an organizational system. • Based on increasing atomic mass and similar chemical properties
Dimitri Medeleev • Predicted the existence of unknown elements • Some elements were out of order https: //encryptedtbn 0. gstatic. com/images? q=tbn: ANd 9 Gc. Td. PYDxt. XWp. Dp. W W-T 2 xb. DAILg 8 GYv. V 0 Sdy. F 3 CIM 3 n 7 UMAT 5370 t 9 g
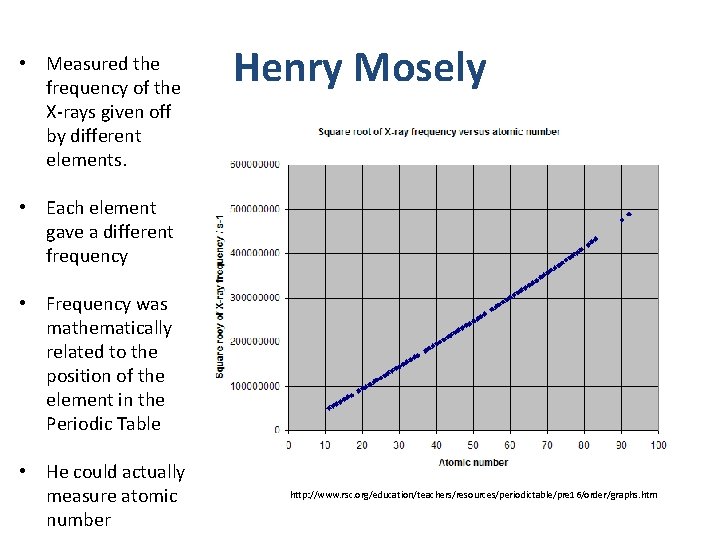
• Measured the frequency of the X-rays given off by different elements. Henry Mosely • Each element gave a different frequency • Frequency was mathematically related to the position of the element in the Periodic Table • He could actually measure atomic number http: //www. rsc. org/education/teachers/resources/periodictable/pre 16/order/graphs. htm

Henry Mosely https: //i. ytimg. com/vi/7 QKA 5 k. Su 9 To/m qdefault. jpg Re- designed the periodic table to show an increase in atomic number. Periodic Table now follows the periodic law.
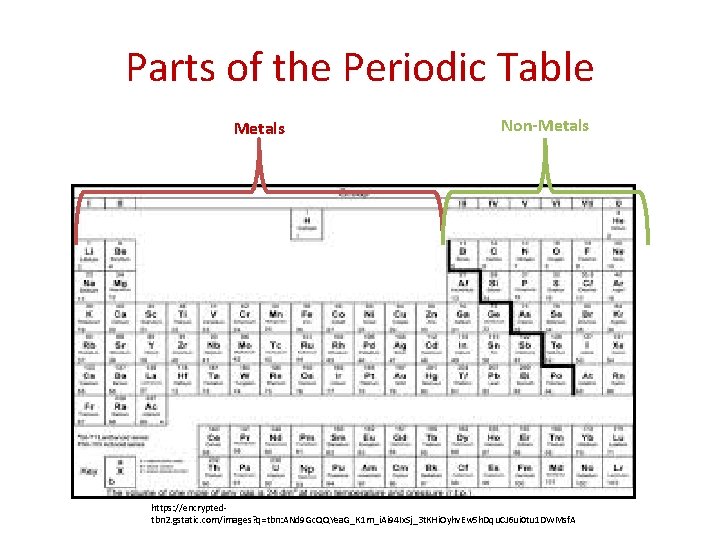
Parts of the Periodic Table Metals Non-Metals https: //encryptedtbn 2. gstatic. com/images? q=tbn: ANd 9 Gc. QQYea. G_K 1 m_i. Ai 94 Ix. Sj_3 t. KHi. Oyhv. Ew 5 h. Dqu. CJ 6 ui 0 tu 1 DWMsf. A

Metals http: //new. testmagic. com/wpcontent/uploads/2012/08/malleable. pn g Ductile https: //encryptedtbn 3. gstatic. com/images? q=tbn: ANd 9 Gc RCd. H 6 G 0 h. On 5 k 4 q 1 Tq 3 XWu. J_SMrh. X 9 w MIk_4 jm 7 j 7 Lz. Ma. M 1 NW_T_CJ 9 iq_O Good conductors of heat and electricity http: //users. ox. ac. uk/~sann 2616/Image s/Good%20 conductor. gif

Metals Are likely to lose electrons, forming positive ions called cations. http: //cdn. hsmemes. com/2013/4/16/560630 dffea 46 c 5 d 81 a c 07 a 3 adadcf 27. jpg

Alkali Metals • Most reactive group of metal elements • All contain 1 valence electron Lithium Metal Click on metal to watch a cool you tube video!

Alkaline Earth Metals • Also a very reactive metal group. • Not as reactive as Alkali metals. http: //www. periodictable. com/Samples/004. 1/s 12 s. JPG
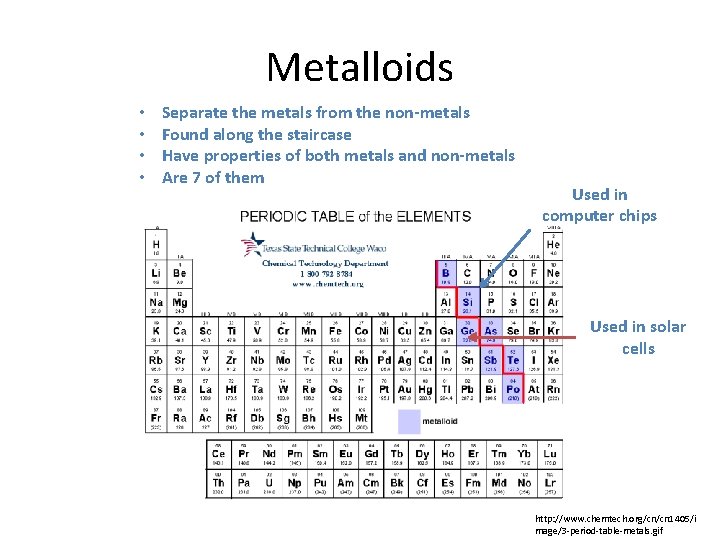
Metalloids • • Separate the metals from the non-metals Found along the staircase Have properties of both metals and non-metals Are 7 of them Used in computer chips Used in solar cells http: //www. chemtech. org/cn/cn 1405/i mage/3 -period-table-metals. gif
Non- Metals • Are brittle, poor conductors of heat and electricity and many are gases at room temperature. • Are likely to gain electrons to form negatively charged ions or anions. 2 S http: //images. sciencedaily. com/2013/02/1302261140 23 -large. jpg

Halogens • Most reactive group of non-metals • Found in Group 17 or VII A • Fluorine is the queen. Chlorine, Bromine and Iodine at room temperature. Fluorine could not be included due to its high reactivity. http: //upload. wikimedia. org/wikipedia/commons/c/c 6/Halogens. jpg
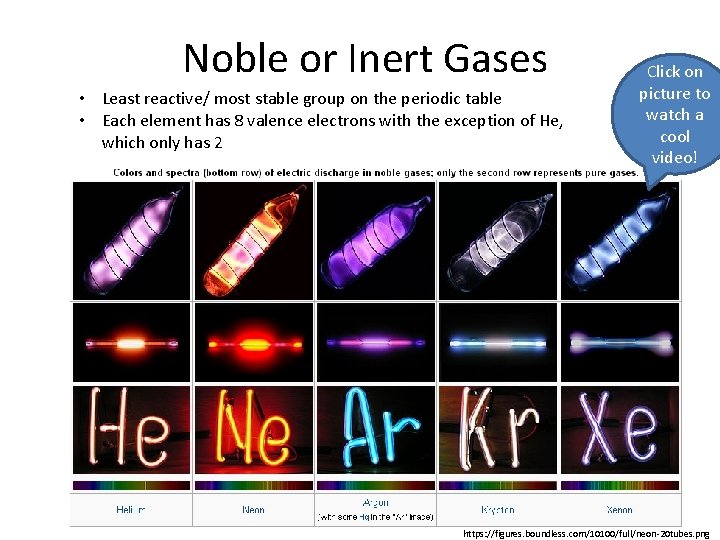
Noble or Inert Gases • Least reactive/ most stable group on the periodic table • Each element has 8 valence electrons with the exception of He, which only has 2 Click on picture to watch a cool video! https: //figures. boundless. com/10100/full/neon-20 tubes. png
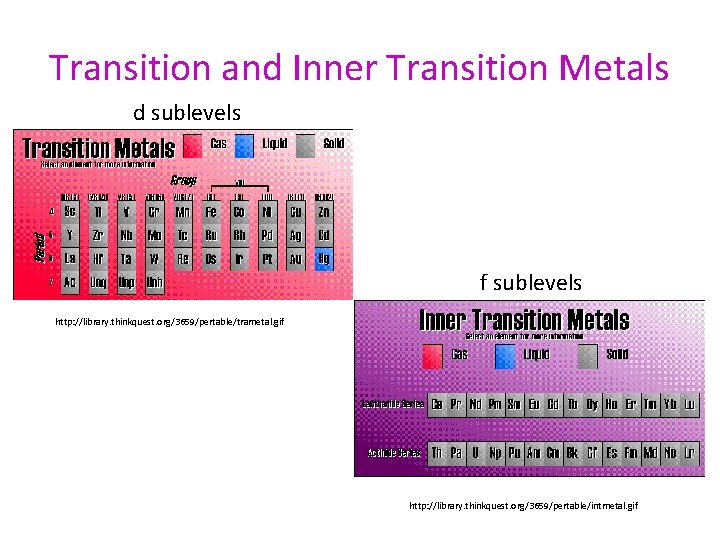
Transition and Inner Transition Metals d sublevels f sublevels http: //library. thinkquest. org/3659/pertable/trametal. gif http: //library. thinkquest. org/3659/pertable/intmetal. gif
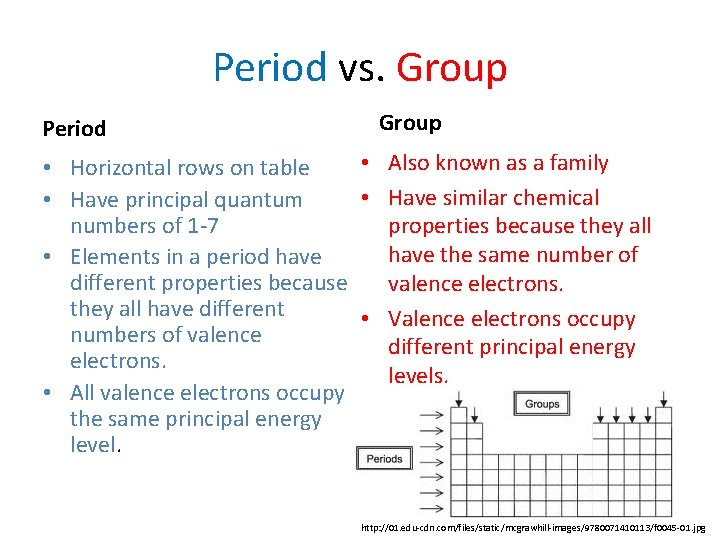
Period vs. Group Period Group • Also known as a family • Horizontal rows on table • Have similar chemical • Have principal quantum numbers of 1 -7 properties because they all have the same number of • Elements in a period have different properties because valence electrons. they all have different • Valence electrons occupy numbers of valence different principal energy electrons. levels. • All valence electrons occupy the same principal energy level. http: //01. edu-cdn. com/files/static/mcgrawhill-images/9780071410113/f 0045 -01. jpg

Numbering the Periodic Table Old Way New Way • A groups and B groups • Numbering the periodic table groups from 1 -18 • Makes all periodic tables uniform • Slightly more difficult to determine valence electrons. (must subtract 10 from groups 13 -18) – B groups were the transition elements – A groups were the rest • Used Roman Numerals to indicate the number of valence electrons for the A groups • Valence electrons are important for bonding /chemical properties • All elements want 8 valence electrons
- Slides: 18