NOTES CHEMICAL REACTIONS PRODUCT substance formed during a

NOTES CHEMICAL REACTIONS: PRODUCT: substance formed during a chemical reaction (right side of arrow) REACTANT: starting substance(s) in a chemical reaction (left side of arrow) Law of Conservation of Mass must be satisfied!

Evidence of Chemical Reactions Temperature change: ✔ endothermic (colder), exothermic (hotter) Color Change ✔Odor ✔Gas Produced (bubbles) ✔Precipitate: formed from 2 ✔ liquids

Balancing Equations Steps 1)Balance atoms that appear only once on each side. 2)Balance polyatomic ions that appear on both sides as a single unit. 3)Balance hydrogens. 4)Balance oxygens. 5)Never change the subscripts of a compound to balance an equation.

Types of Reactions Synthesis Reaction: 1. Two or more substances combine to form a single compound. 2. Usually energy is released (exothermic) 3. Basic reaction: A + B --> AB
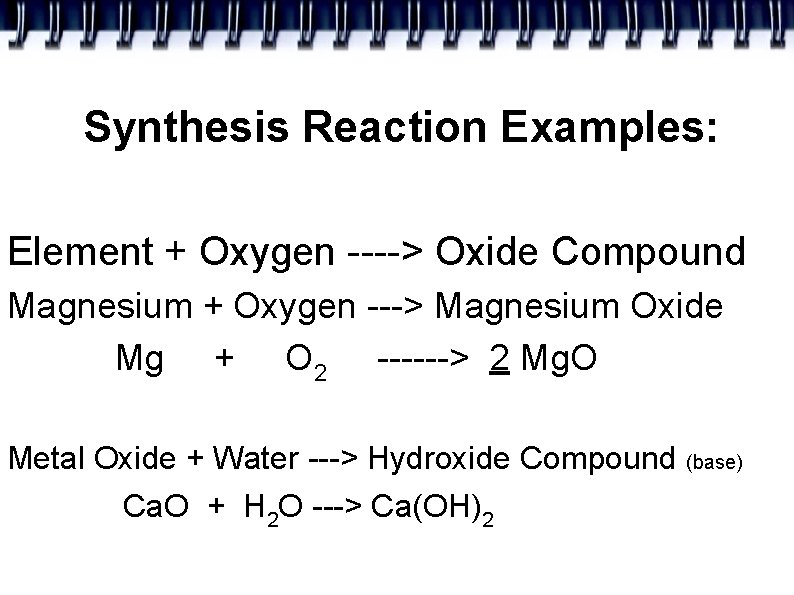
Synthesis Reaction Examples: Element + Oxygen ----> Oxide Compound Magnesium + Oxygen ---> Magnesium Oxide Mg + O 2 ------> 2 Mg. O Metal Oxide + Water ---> Hydroxide Compound (base) Ca. O + H 2 O ---> Ca(OH)2
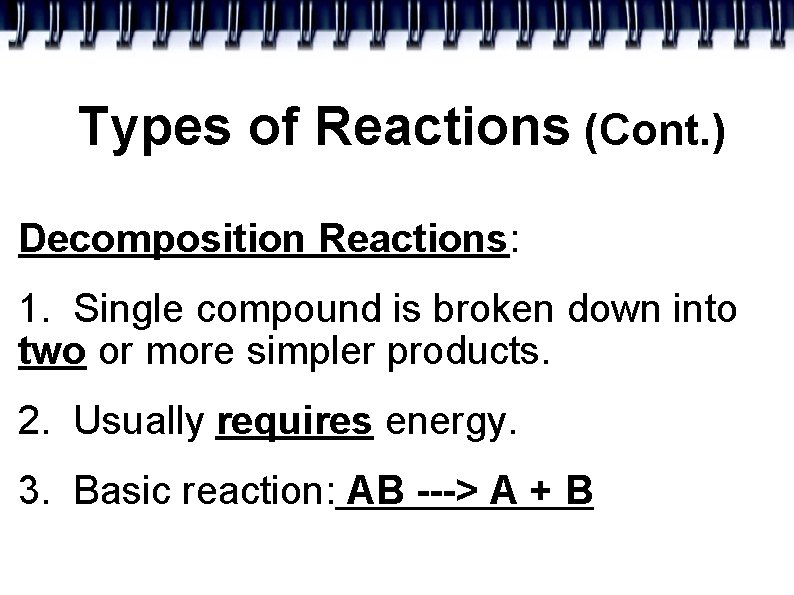
Types of Reactions (Cont. ) Decomposition Reactions: 1. Single compound is broken down into two or more simpler products. 2. Usually requires energy. 3. Basic reaction: AB ---> A + B
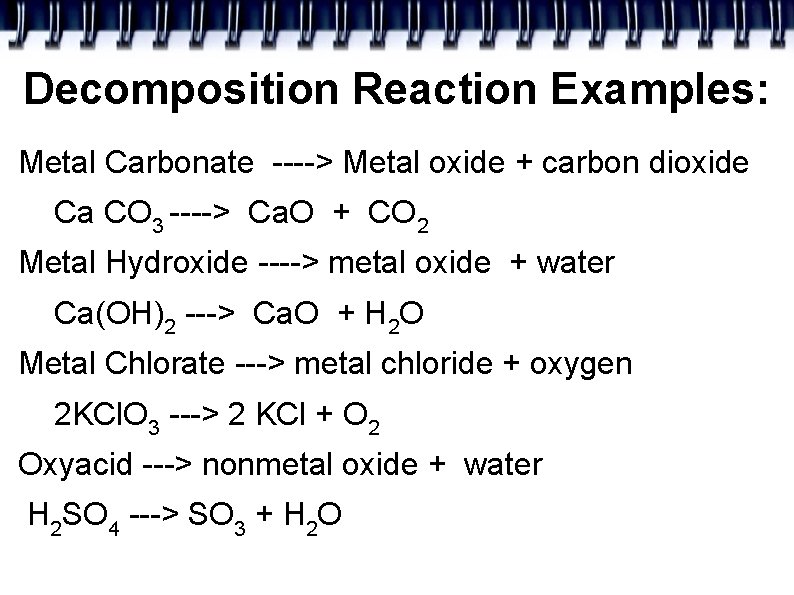
Decomposition Reaction Examples: Metal Carbonate ----> Metal oxide + carbon dioxide Ca CO 3 ----> Ca. O + CO 2 Metal Hydroxide ----> metal oxide + water Ca(OH)2 ---> Ca. O + H 2 O Metal Chlorate ---> metal chloride + oxygen 2 KCl. O 3 ---> 2 KCl + O 2 Oxyacid ---> nonmetal oxide + water H 2 SO 4 ---> SO 3 + H 2 O

Types of Reactions (cont. ) SINGLE REPLACEMENT REACTION: 1. One element replaces a similar element in a compound. 2. A reactive metal will replace any metal that is less reactive (see pg 288 Activity Series of Metals) 3. Nonmetal will replace other nonmetals.
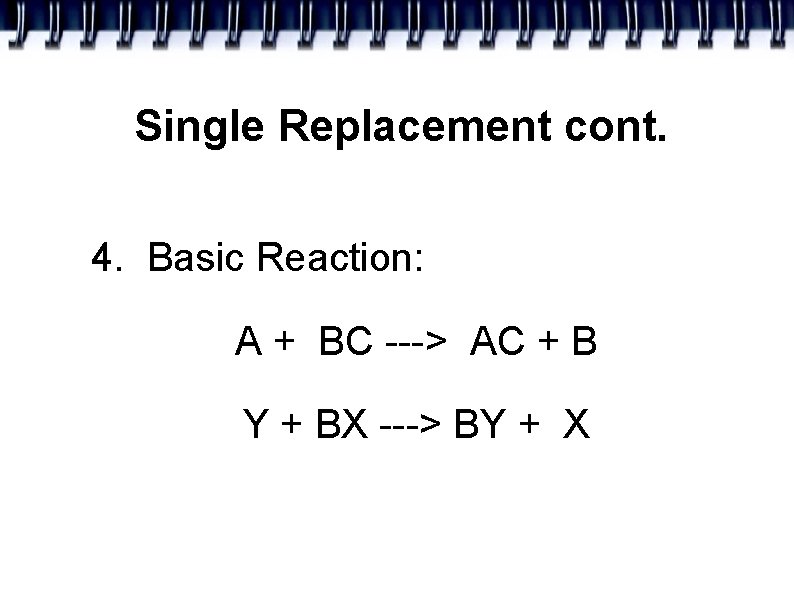
Single Replacement cont. 4. Basic Reaction: A + BC ---> AC + B Y + BX ---> BY + X
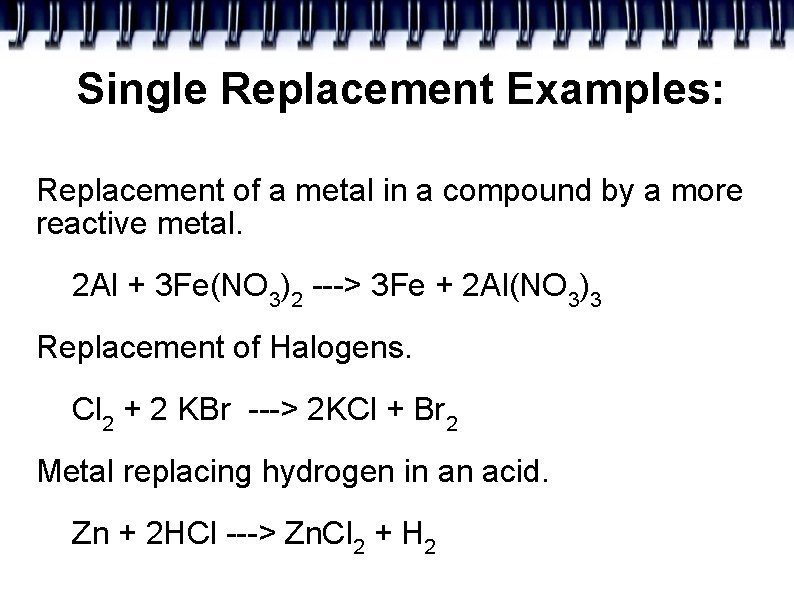
Single Replacement Examples: Replacement of a metal in a compound by a more reactive metal. 2 Al + 3 Fe(NO 3)2 ---> 3 Fe + 2 Al(NO 3)3 Replacement of Halogens. Cl 2 + 2 KBr ---> 2 KCl + Br 2 Metal replacing hydrogen in an acid. Zn + 2 HCl ---> Zn. Cl 2 + H 2
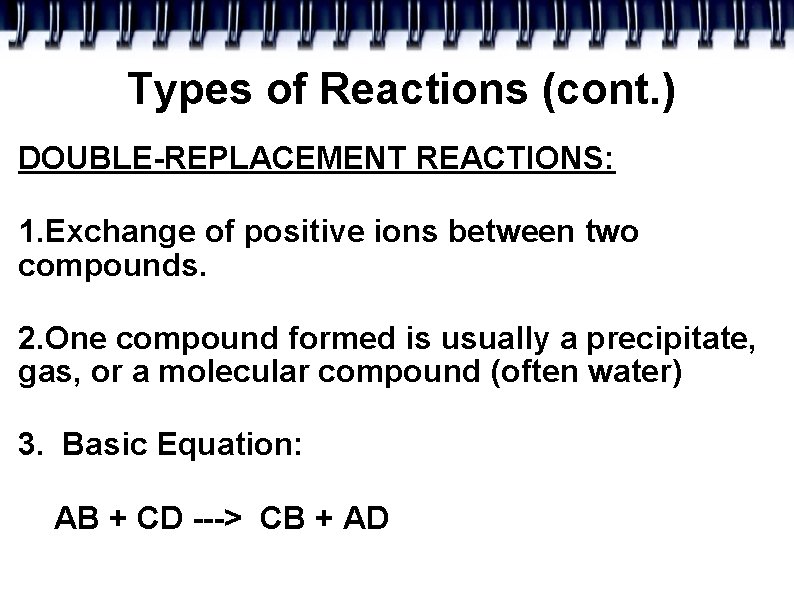
Types of Reactions (cont. ) DOUBLE-REPLACEMENT REACTIONS: 1. Exchange of positive ions between two compounds. 2. One compound formed is usually a precipitate, gas, or a molecular compound (often water) 3. Basic Equation: AB + CD ---> CB + AD
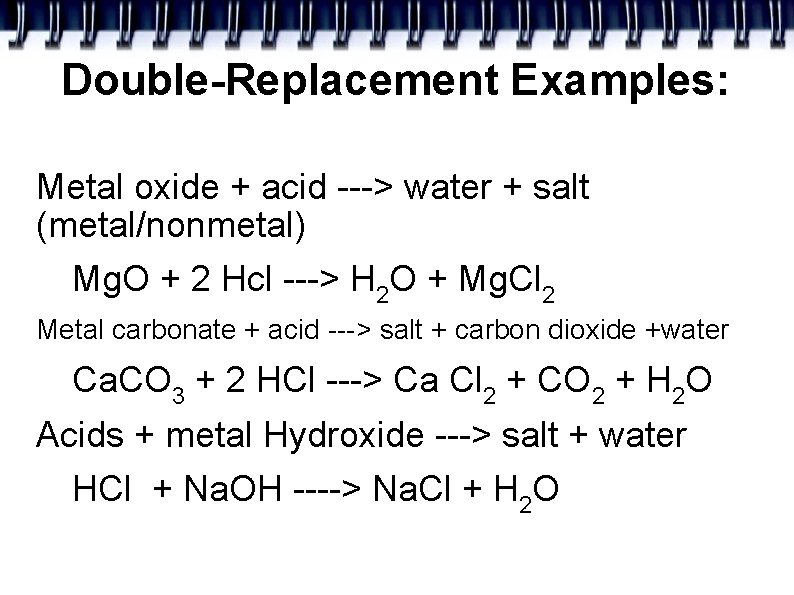
Double-Replacement Examples: Metal oxide + acid ---> water + salt (metal/nonmetal) Mg. O + 2 Hcl ---> H 2 O + Mg. Cl 2 Metal carbonate + acid ---> salt + carbon dioxide +water Ca. CO 3 + 2 HCl ---> Ca Cl 2 + CO 2 + H 2 O Acids + metal Hydroxide ---> salt + water HCl + Na. OH ----> Na. Cl + H 2 O
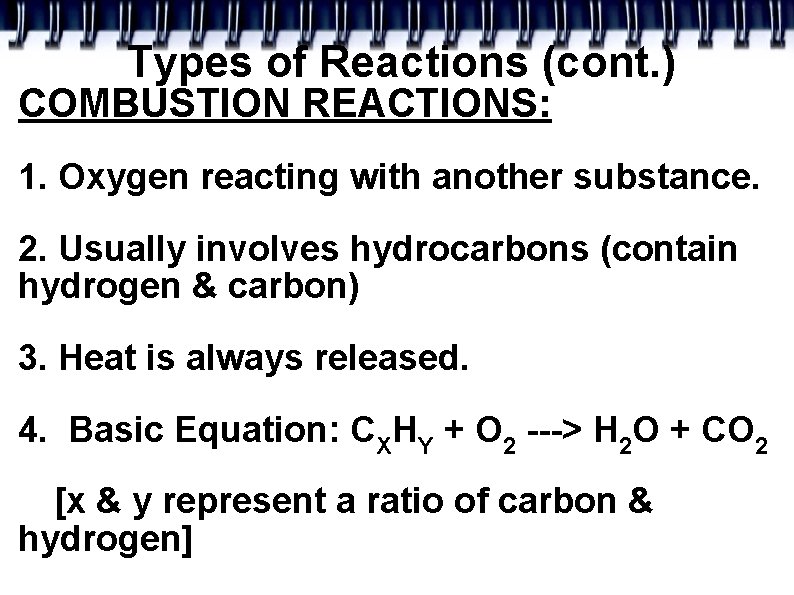
Types of Reactions (cont. ) COMBUSTION REACTIONS: 1. Oxygen reacting with another substance. 2. Usually involves hydrocarbons (contain hydrogen & carbon) 3. Heat is always released. 4. Basic Equation: CXHY + O 2 ---> H 2 O + CO 2 [x & y represent a ratio of carbon & hydrogen]
Combustion Examples: 4. Complete combustion: C 3 H 8 + 5 O 2 ---> 3 CO 2 + 4 H 2 O 5. Incomplete combustion: creates carbon monoxide (CO), carbon, & water. [products cannot be predicted]
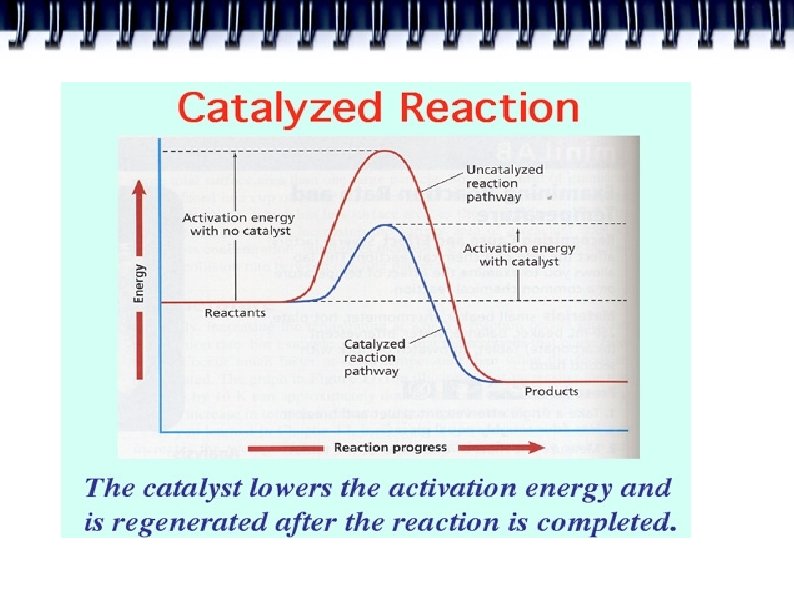
Catalysts A substance that increases the rate of a chemical reaction by lowering activation energies but is not itself consumed in the reaction. Example: Enzymes: allow many chemical rxns to occur at a rate that sustains life at normal living temperatures
- Slides: 16