Notes 79 A Ionic and Molecular Compounds Writing







- Slides: 25

Notes 7&9 A Ionic and Molecular Compounds Writing Formulas and Naming

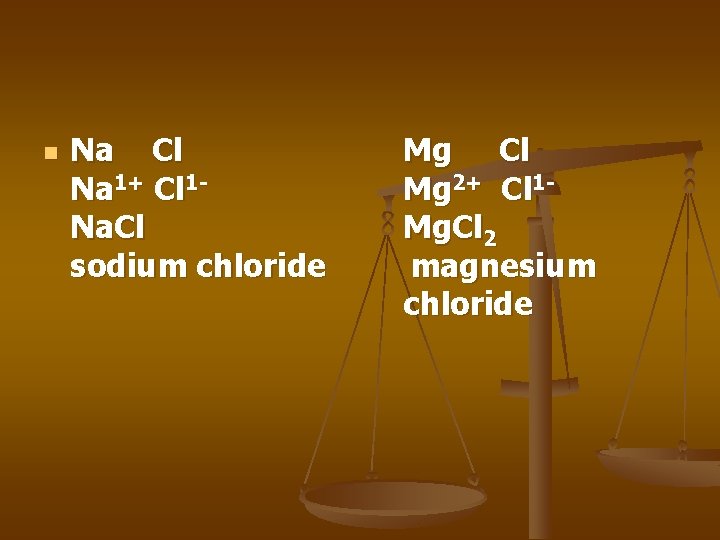
n Na Cl Na 1+ Cl 1 Na. Cl sodium chloride Mg Cl Mg 2+ Cl 1 Mg. Cl 2 magnesium chloride

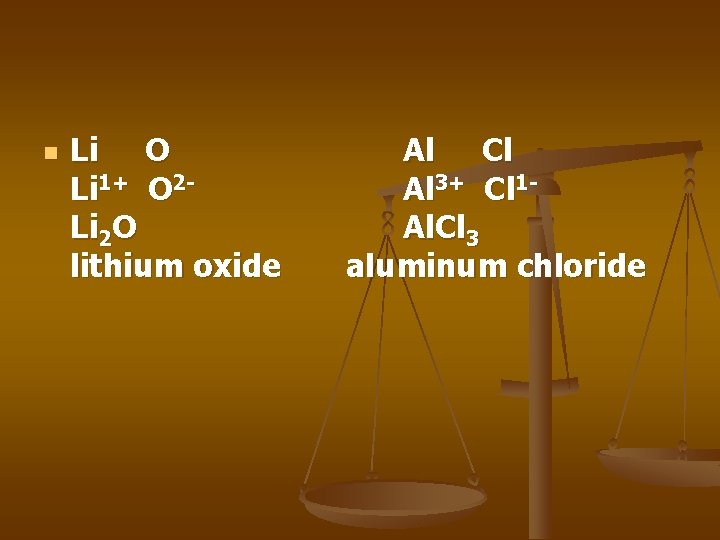
n Li O Li 1+ O 2 Li 2 O lithium oxide Al Cl Al 3+ Cl 1 Al. Cl 3 aluminum chloride

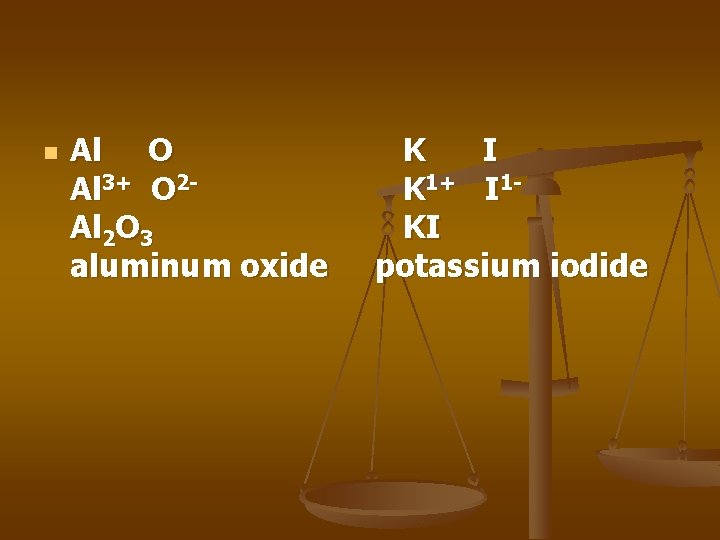
n Al O Al 3+ O 2 Al 2 O 3 aluminum oxide K I K 1+ I 1 KI potassium iodide

n n Ca Br Ca 2+ Br 1 Ca. Br 2 calcium bromide Ga S Ga 3+ S 2 Ga 2 S 3 gallium sulfide

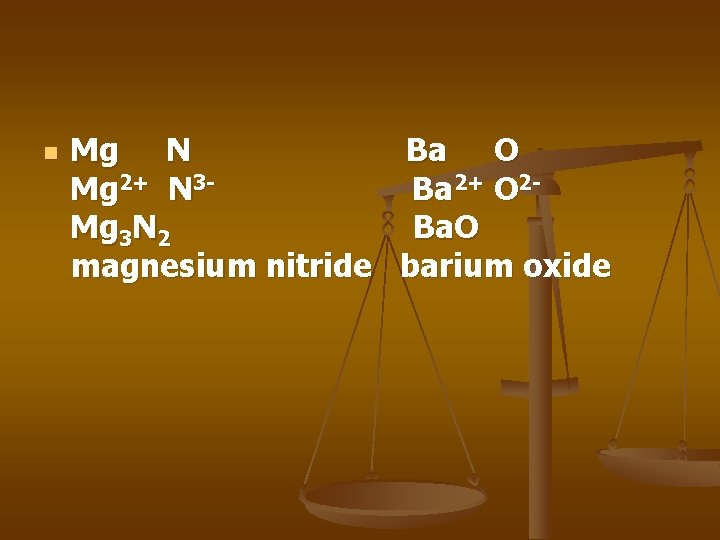
n Mg N Ba O Mg 2+ N 3 Ba 2+ O 2 Mg 3 N 2 Ba. O magnesium nitride barium oxide

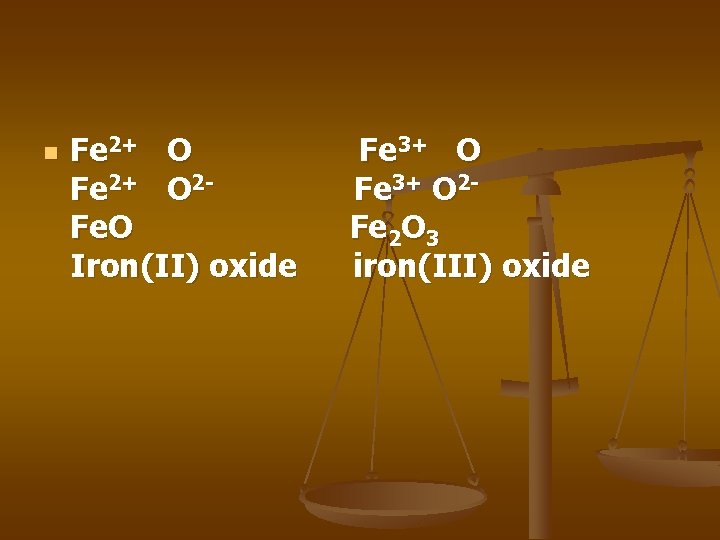
n Fe 2+ O 2 Fe. O Iron(II) oxide Fe 3+ O 2 Fe 2 O 3 iron(III) oxide

Notes 7&9 B n n n K 1+ NO 31 KNO 3 potassium nitrate Na 1+ SO 32 Na 2 SO 3 sodium sulfite

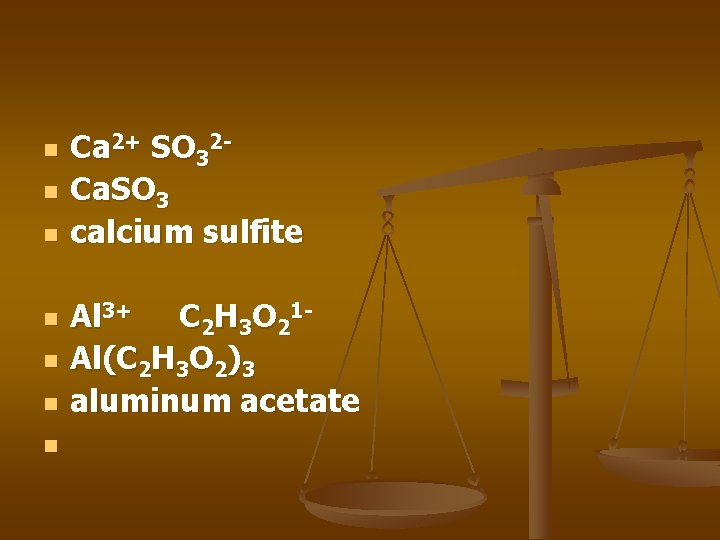
n n n n Ca 2+ SO 32 Ca. SO 3 calcium sulfite Al 3+ C 2 H 3 O 21 Al(C 2 H 3 O 2)3 aluminum acetate

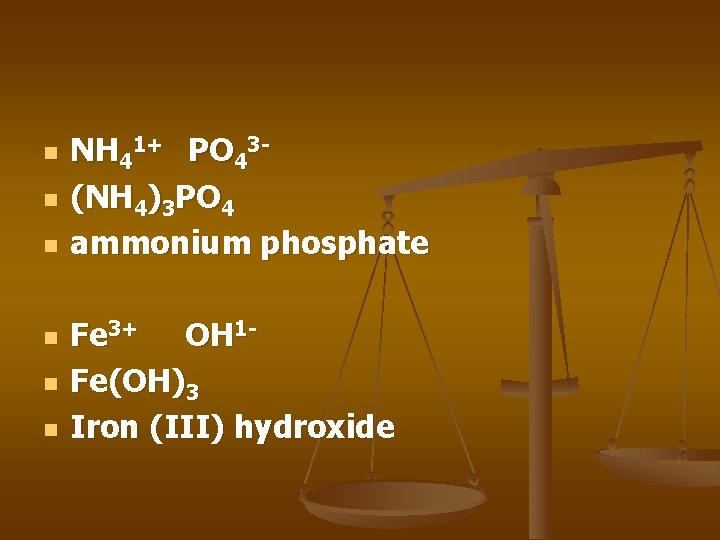
n n n NH 41+ PO 43(NH 4)3 PO 4 ammonium phosphate Fe 3+ OH 1 Fe(OH)3 Iron (III) hydroxide

Notes 7&9 C n n Name Ions Formula lithium oxide Li 1+ O 2 Li 2 O strontium fluoride Sr 2+ F 1 Sr. F 2

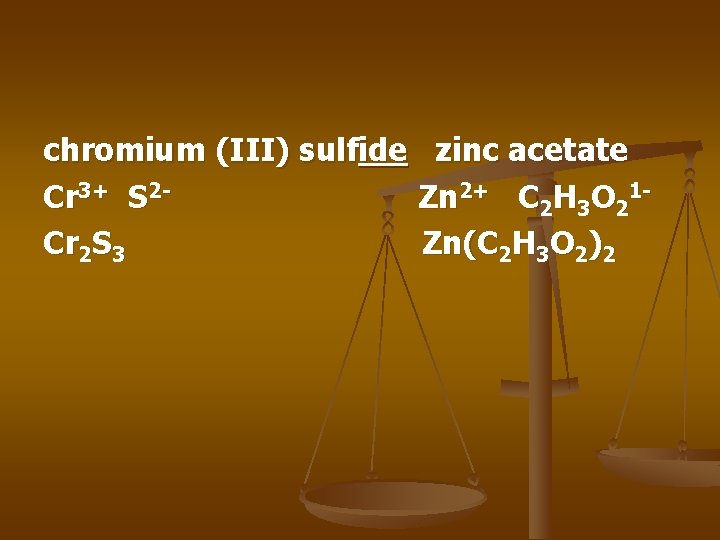
chromium (III) sulfide zinc acetate Cr 3+ S 2 Zn 2+ C 2 H 3 O 21 Cr 2 S 3 Zn(C 2 H 3 O 2)2

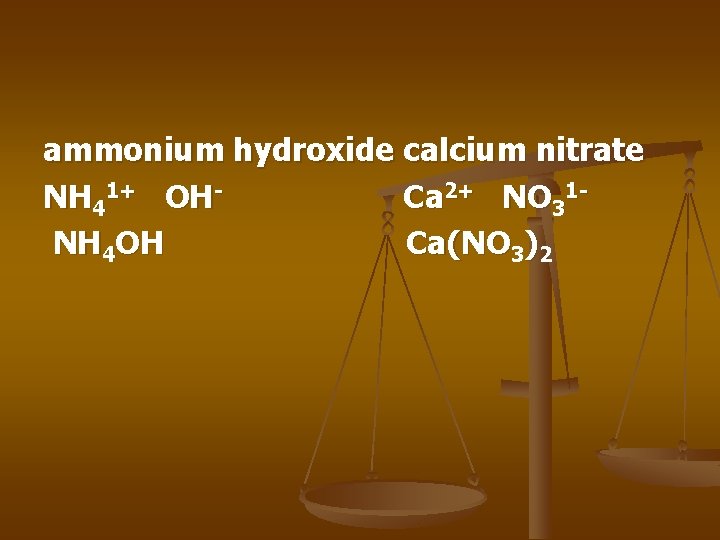
ammonium hydroxide calcium nitrate NH 41+ OHCa 2+ NO 31 NH 4 OH Ca(NO 3)2

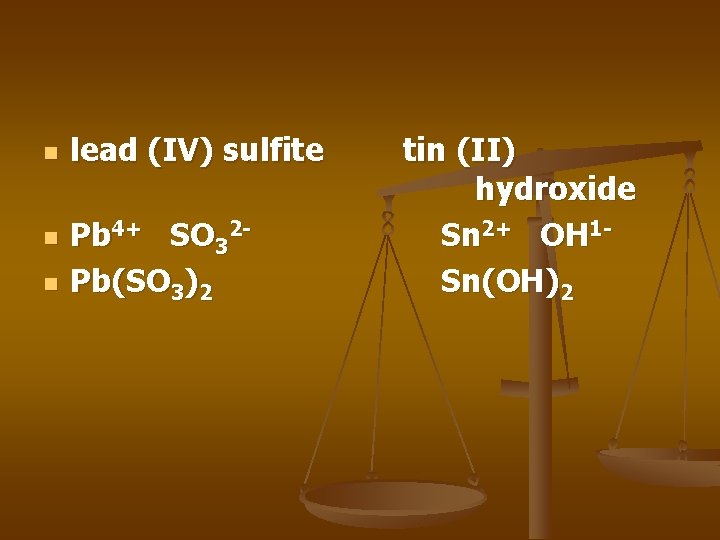
n n n lead (IV) sulfite Pb 4+ SO 32 Pb(SO 3)2 tin (II) hydroxide Sn 2+ OH 1 Sn(OH)2

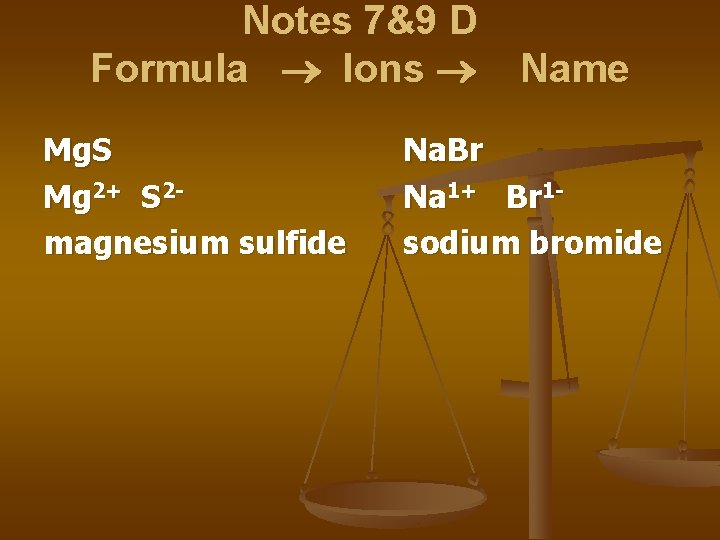
Notes 7&9 D Formula Ions Mg. S Mg 2+ S 2 magnesium sulfide Name Na. Br Na 1+ Br 1 sodium bromide

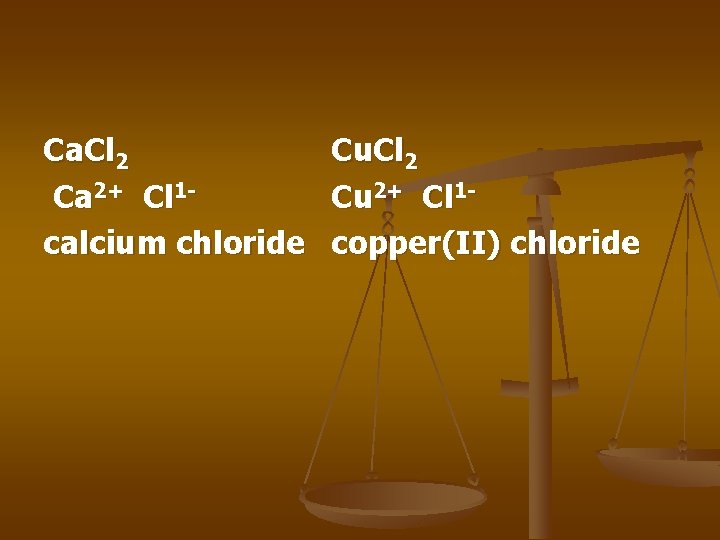
Ca. Cl 2 Ca 2+ Cl 1 calcium chloride Cu. Cl 2 Cu 2+ Cl 1 copper(II) chloride

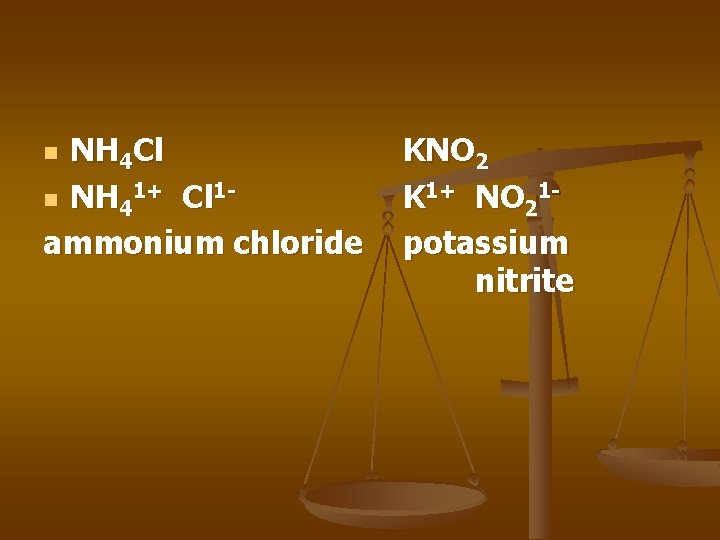
NH 4 Cl n NH 41+ Cl 1 ammonium chloride n KNO 2 K 1+ NO 21 potassium nitrite

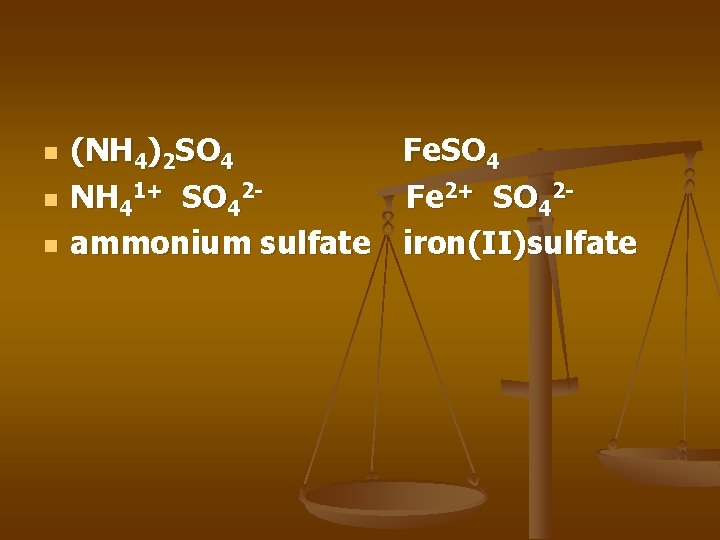
n n n (NH 4)2 SO 4 Fe. SO 4 NH 41+ SO 42 Fe 2+ SO 42 ammonium sulfate iron(II)sulfate

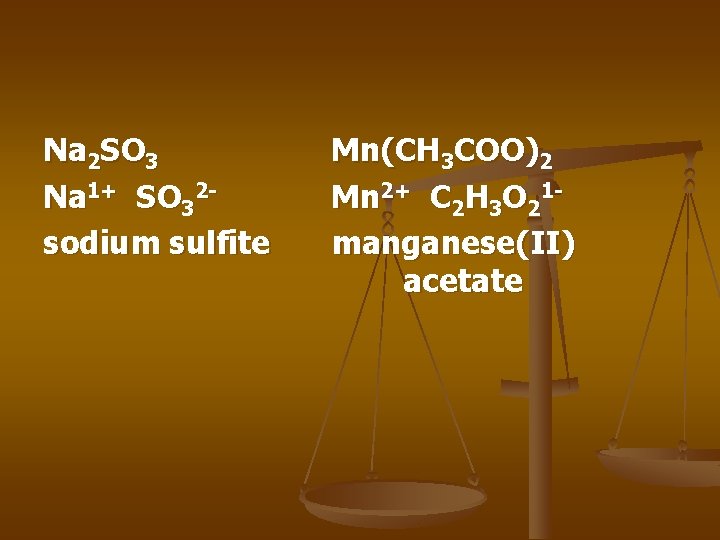
Na 2 SO 3 Na 1+ SO 32 sodium sulfite Mn(CH 3 COO)2 Mn 2+ C 2 H 3 O 21 manganese(II) acetate

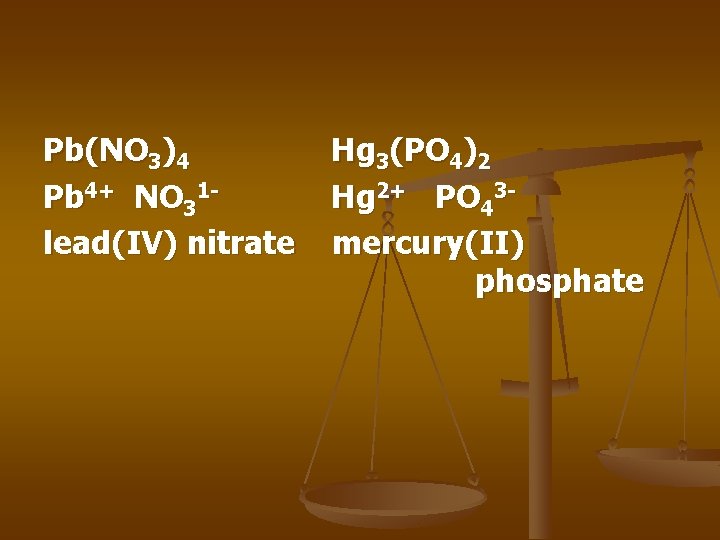
Pb(NO 3)4 Pb 4+ NO 31 lead(IV) nitrate Hg 3(PO 4)2 Hg 2+ PO 43 mercury(II) phosphate

n n Pb. SO 4 HG 3(PO 4)2 Pb 2+ SO 42 HG 2+ PO 43 lead(II)sulfate mercury (II) phosphate

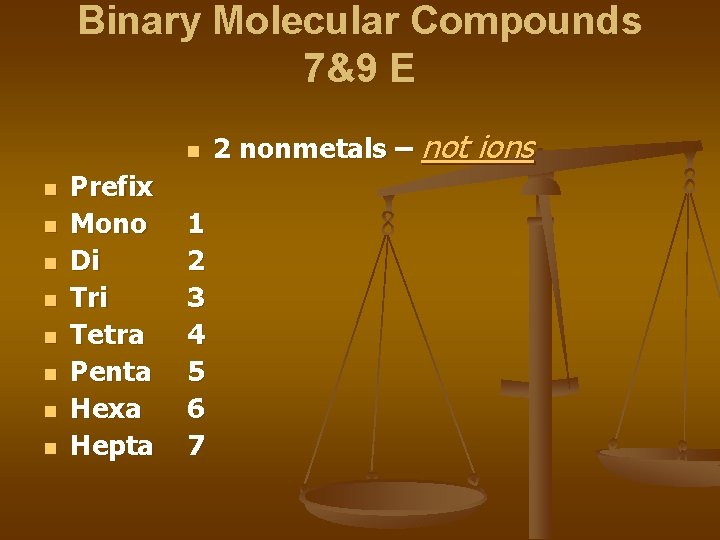
Binary Molecular Compounds 7&9 E n n n n n Prefix Mono Di Tri Tetra Penta Hexa Hepta 1 2 3 4 5 6 7 2 nonmetals – not ions

n n Hepta Octa Nona Deca 7 8 9 10

n N 2 O n n all end in –ide SF 6 n dinitrogen monoxide sulfur hexafloride omit mono when the first element has one atom CO Cl 2 O 8 carbon monoxide dichlorine octoxide

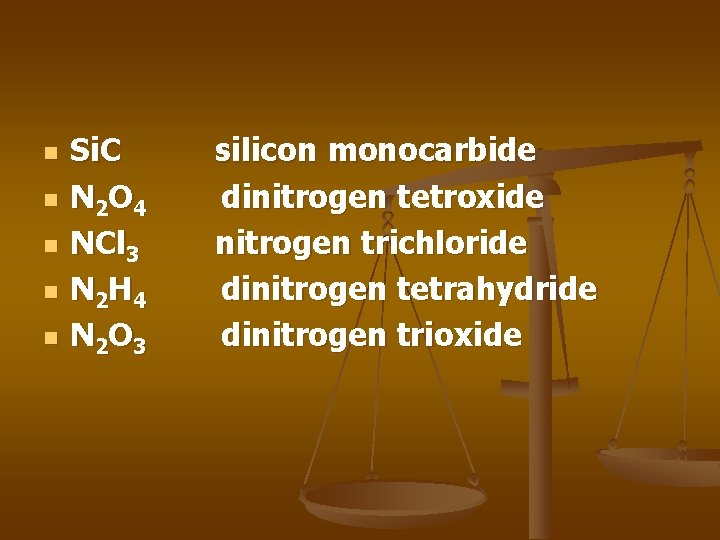
n n n Si. C N 2 O 4 NCl 3 N 2 H 4 N 2 O 3 silicon monocarbide dinitrogen tetroxide nitrogen trichloride dinitrogen tetrahydride dinitrogen trioxide