Notes 7 1 7 2 Matter and Temperature
- Slides: 10

Notes 7. 1& 7. 2: Matter and Temperature

• An element is defined as a pure substance that can’t be broken down into simpler substances by physical or chemical means. • Examples include: hydrogen, oxygen, gold, mercury.

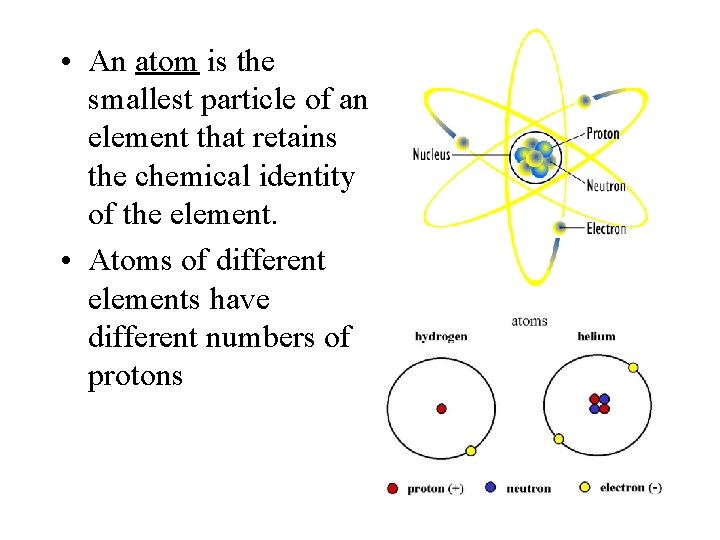
• An atom is the smallest particle of an element that retains the chemical identity of the element. • Atoms of different elements have different numbers of protons

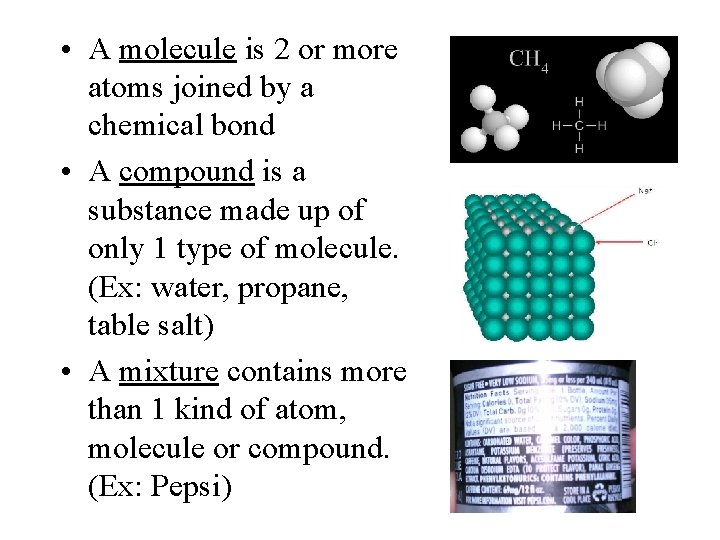
• A molecule is 2 or more atoms joined by a chemical bond • A compound is a substance made up of only 1 type of molecule. (Ex: water, propane, table salt) • A mixture contains more than 1 kind of atom, molecule or compound. (Ex: Pepsi)

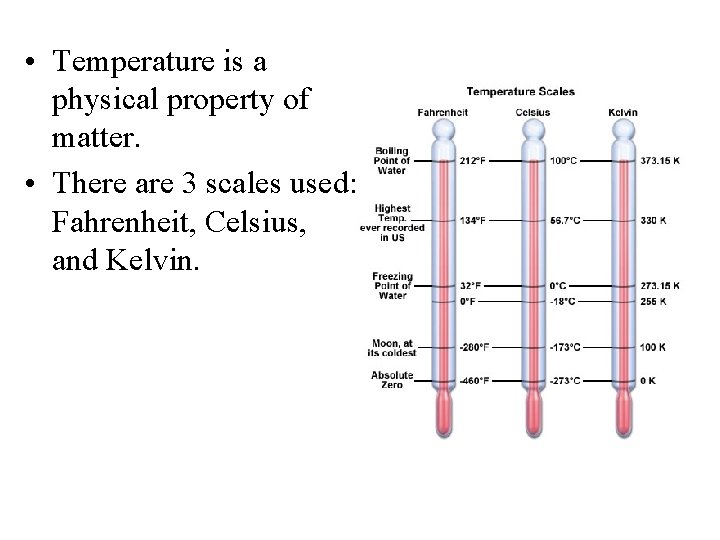
• Temperature is a physical property of matter. • There are 3 scales used: Fahrenheit, Celsius, and Kelvin.

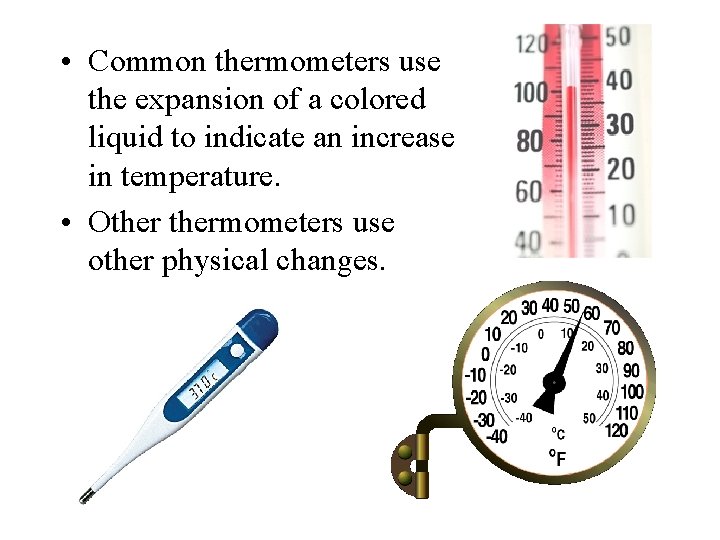
• Common thermometers use the expansion of a colored liquid to indicate an increase in temperature. • Othermometers use other physical changes.

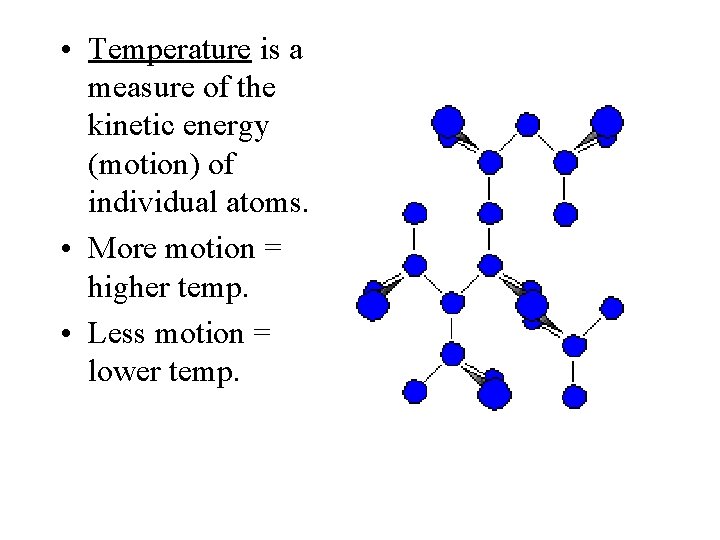
• Temperature is a measure of the kinetic energy (motion) of individual atoms. • More motion = higher temp. • Less motion = lower temp.

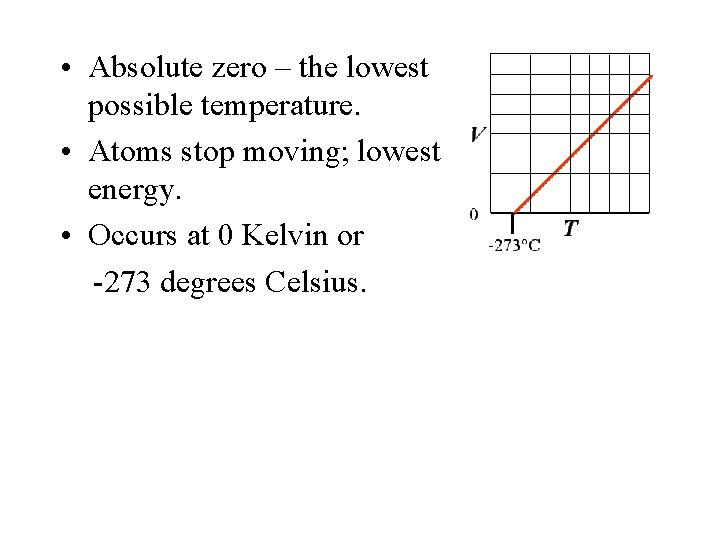
• Absolute zero – the lowest possible temperature. • Atoms stop moving; lowest energy. • Occurs at 0 Kelvin or -273 degrees Celsius.

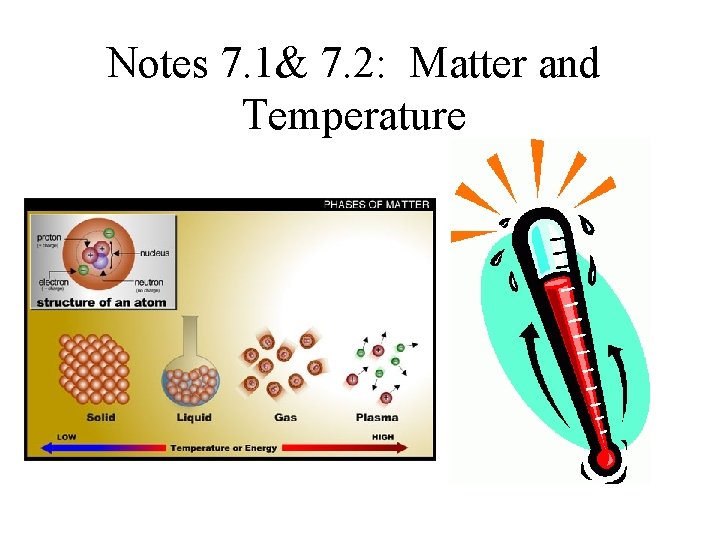
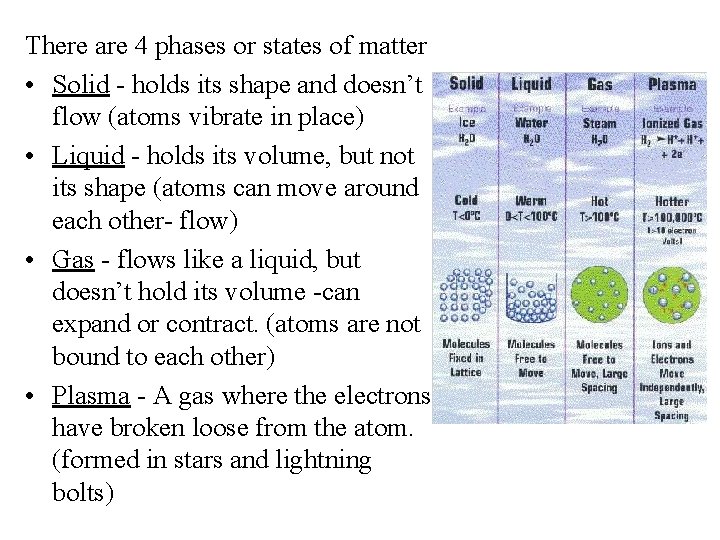
There are 4 phases or states of matter • Solid - holds its shape and doesn’t flow (atoms vibrate in place) • Liquid - holds its volume, but not its shape (atoms can move around each other- flow) • Gas - flows like a liquid, but doesn’t hold its volume -can expand or contract. (atoms are not bound to each other) • Plasma - A gas where the electrons have broken loose from the atom. (formed in stars and lightning bolts)

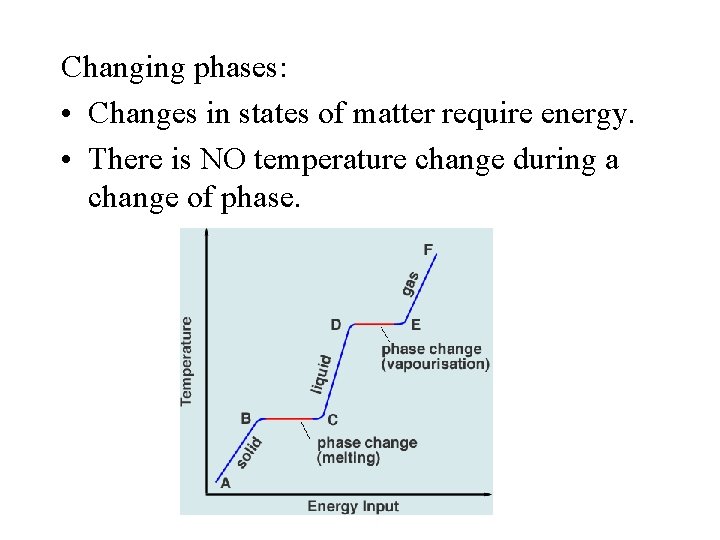
Changing phases: • Changes in states of matter require energy. • There is NO temperature change during a change of phase.