Notes 3 Chemical Bonds Isotopes Ions Bonding properties

Notes 3 - Chemical Bonds, Isotopes, Ions
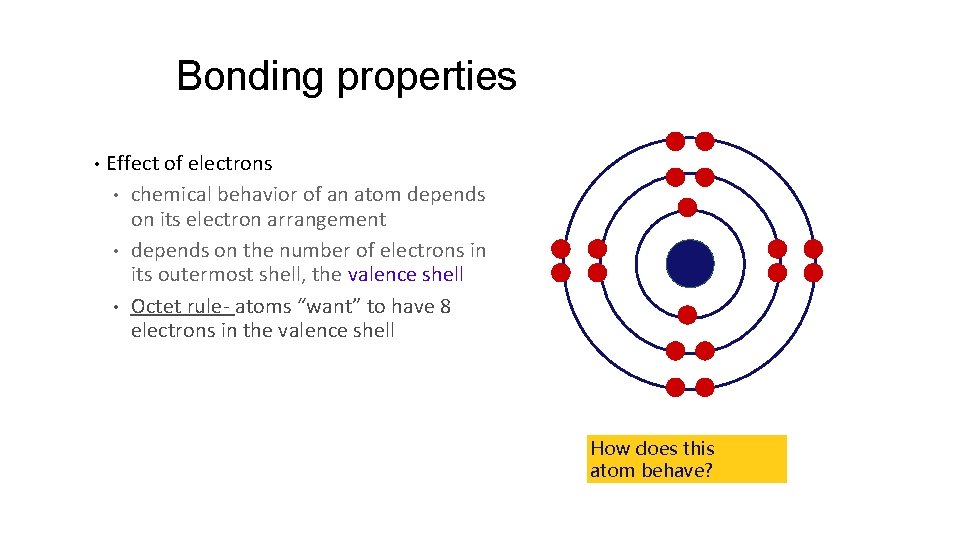
Bonding properties • Effect of electrons • chemical behavior of an atom depends on its electron arrangement • depends on the number of electrons in its outermost shell, the valence shell • Octet rule- atoms “want” to have 8 electrons in the valence shell How does this atom behave?
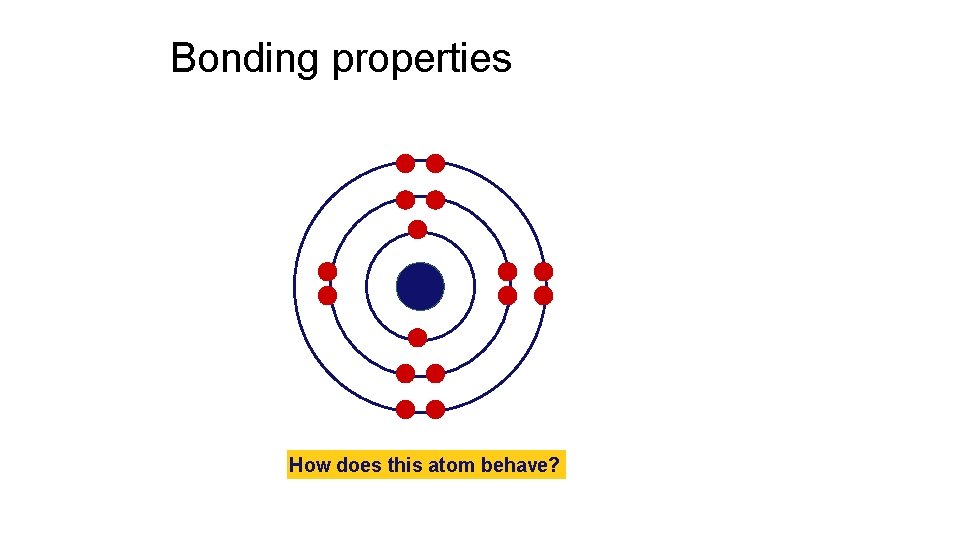
Bonding properties How does this atom behave?

Chemical reactivity • Atoms tend to: • Complete a partially filled outer (valence) electron shell or • Empty a partially filled outer (valence) electron shell • 8 is the magic number of valence electrons!! • This tendency drives chemical reactions
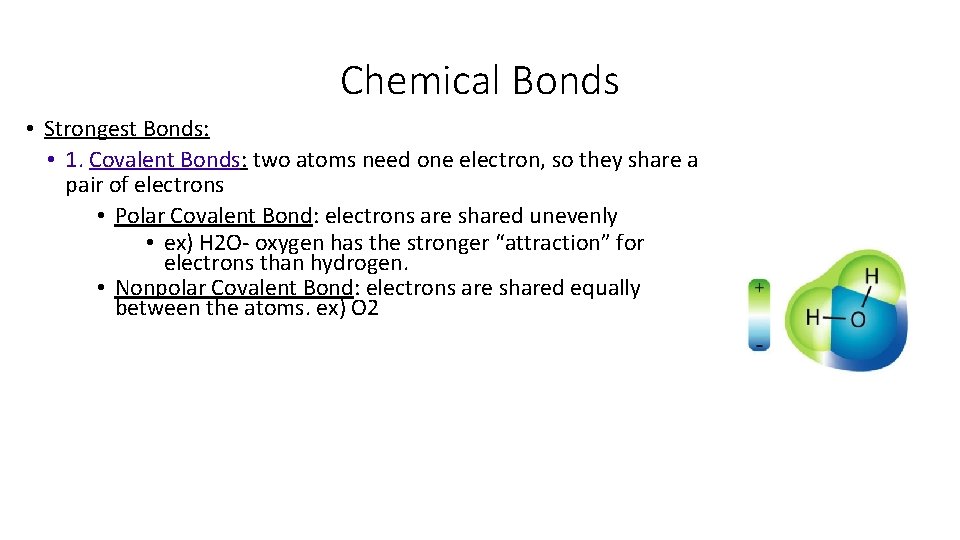
Chemical Bonds • Strongest Bonds: • 1. Covalent Bonds: two atoms need one electron, so they share a pair of electrons • Polar Covalent Bond: electrons are shared unevenly • ex) H 2 O- oxygen has the stronger “attraction” for electrons than hydrogen. • Nonpolar Covalent Bond: electrons are shared equally between the atoms. ex) O 2
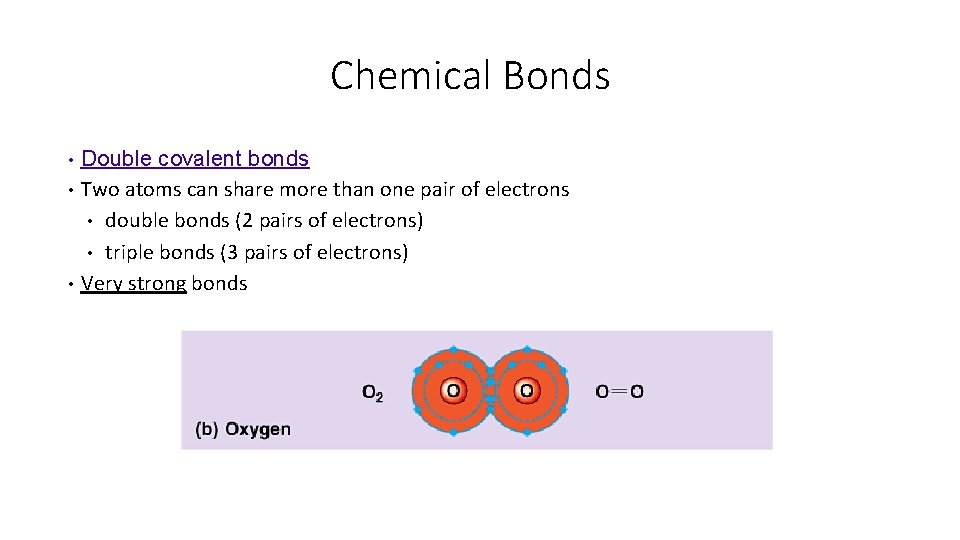
Chemical Bonds • • • Double covalent bonds Two atoms can share more than one pair of electrons • double bonds (2 pairs of electrons) • triple bonds (3 pairs of electrons) Very strong bonds
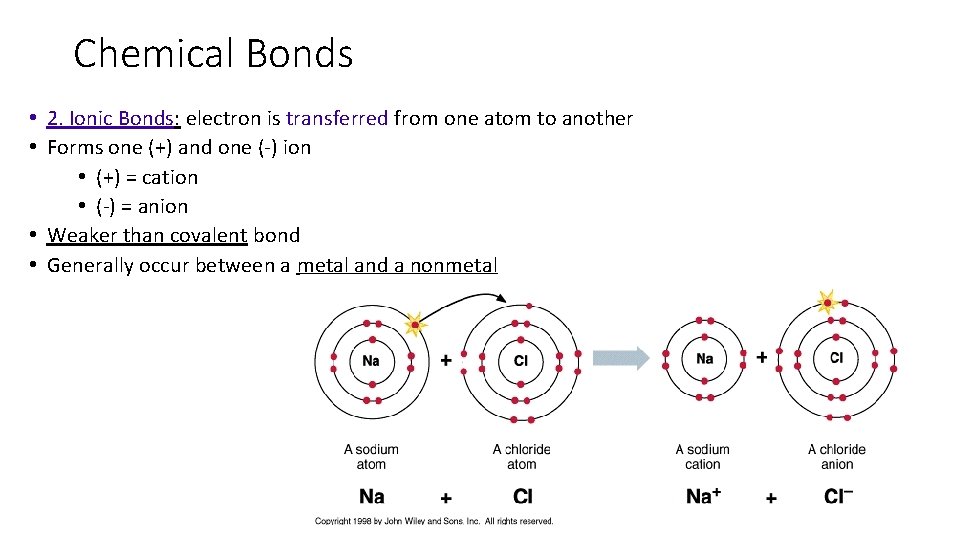
Chemical Bonds • 2. Ionic Bonds: electron is transferred from one atom to another • Forms one (+) and one (-) ion • (+) = cation • (-) = anion • Weaker than covalent bond • Generally occur between a metal and a nonmetal

Isotopes • # of neutrons varies, but same # of protons (same element, different mass) • Radioactive isotopes used as biological tracers (follow molecules, medical diagnoses) • Some are unstable- can also be a biological hazard
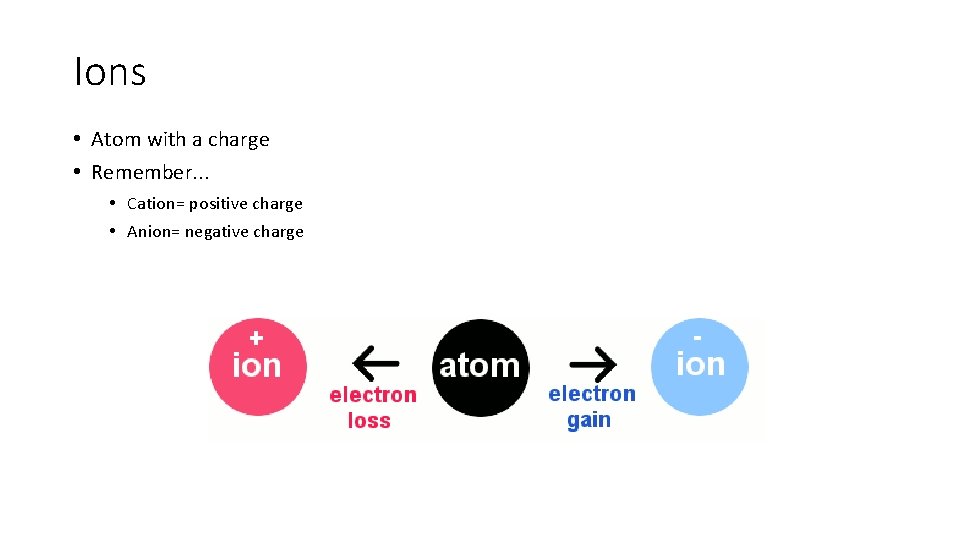
Ions • Atom with a charge • Remember. . . • Cation= positive charge • Anion= negative charge
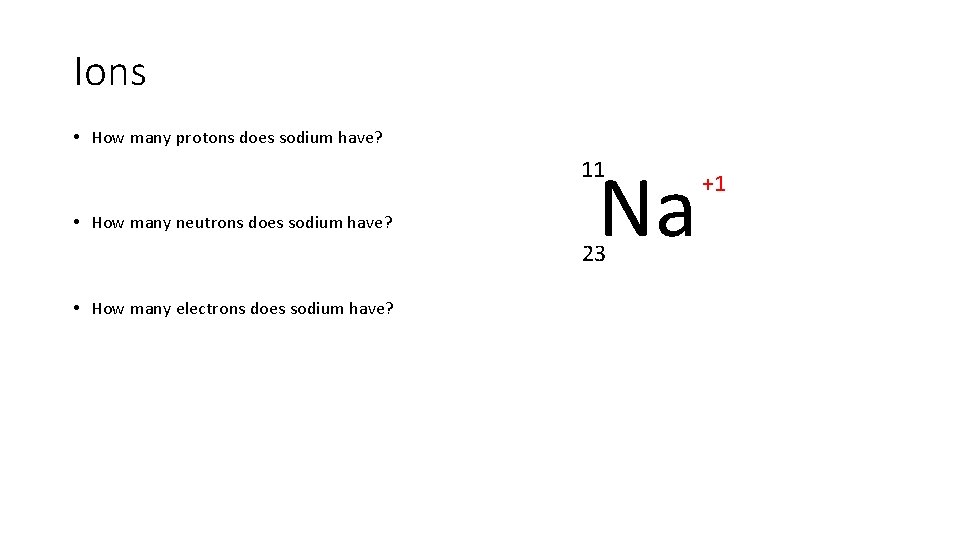
Ions • How many protons does sodium have? Na 11 • How many neutrons does sodium have? 23 • How many electrons does sodium have? +1
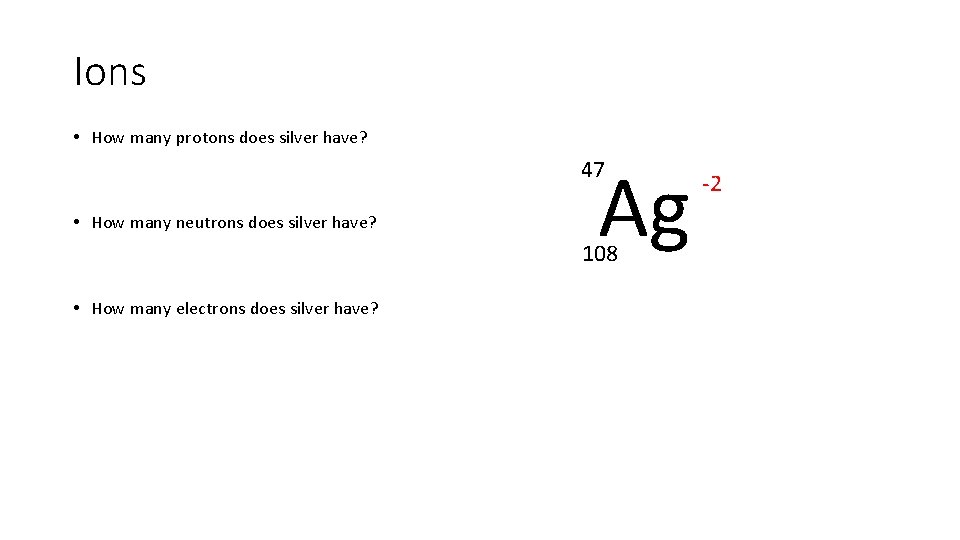
Ions • How many protons does silver have? Ag 47 • How many neutrons does silver have? 108 • How many electrons does silver have? -2
- Slides: 11