NOTES 2 3 part 2 Nucleic Acids Proteins



- Slides: 41

NOTES: 2. 3 part 2 Nucleic Acids & Proteins

So far, we’ve covered… the following MACROMOLECULES: ● CARBOHYDRATES… ● LIPIDS… Let’s review…

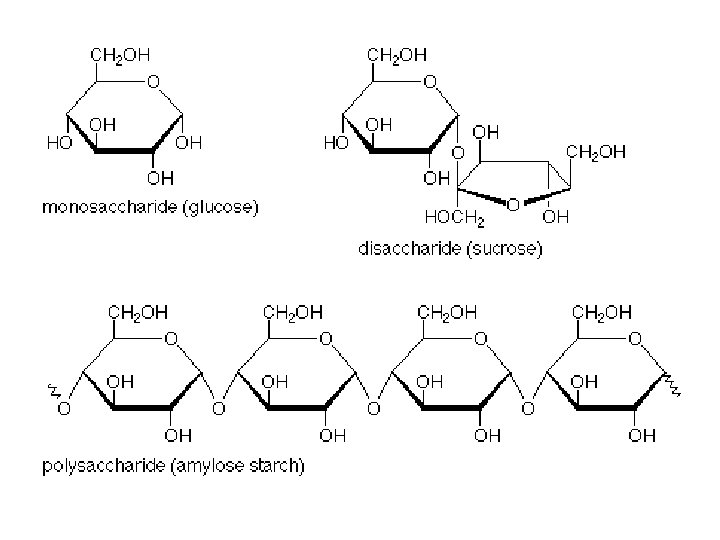
CARBOHYDRATES: ● elements? ● purpose(s)? ● monomers / building blocks? ● polymers? ● examples?

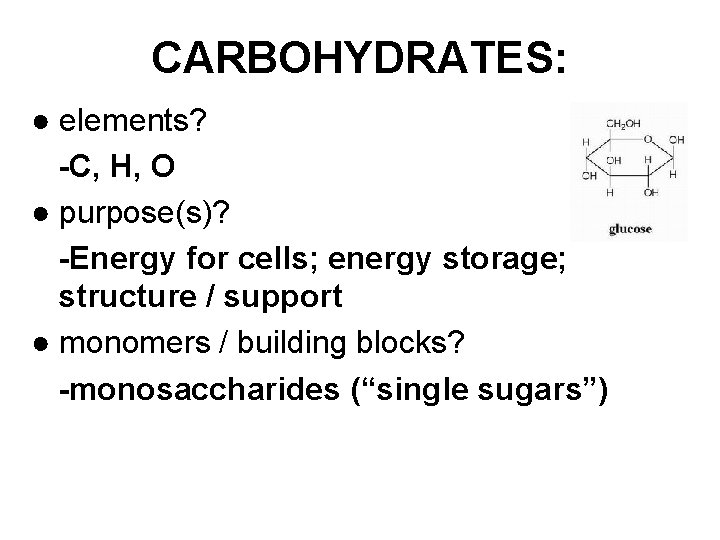
CARBOHYDRATES: ● elements? -C, H, O ● purpose(s)? -Energy for cells; energy storage; structure / support ● monomers / building blocks? -monosaccharides (“single sugars”)

CARBOHYDRATES: ● polymers? -polysaccharides ● examples? -MONO: glucose, fructose, ribose -DI: sucrose, maltose -POLY: starch, cellulose, glycogen


LIPIDS: ● elements? ● purpose(s)? ● monomers / building blocks? ● polymers? ● examples?

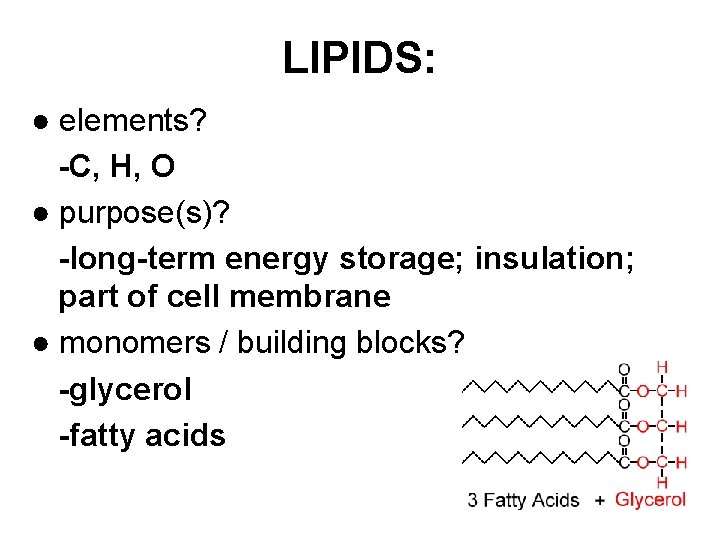
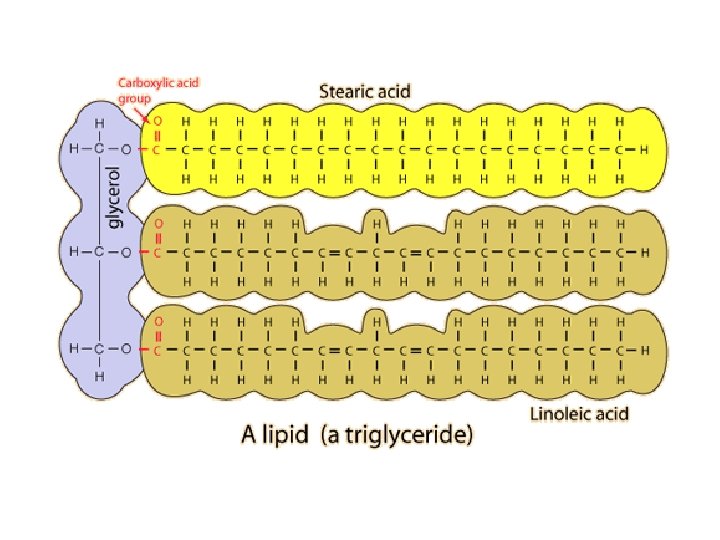
LIPIDS: ● elements? -C, H, O ● purpose(s)? -long-term energy storage; insulation; part of cell membrane ● monomers / building blocks? -glycerol -fatty acids

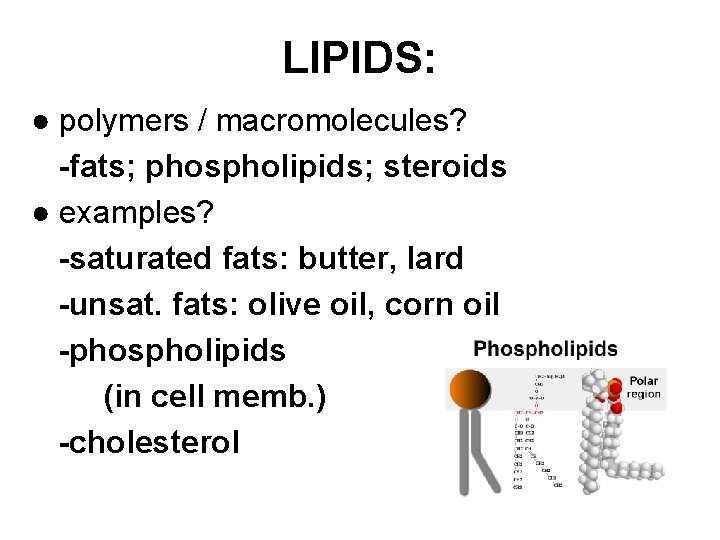
LIPIDS: ● polymers / macromolecules? -fats; phospholipids; steroids ● examples? -saturated fats: butter, lard -unsat. fats: olive oil, corn oil -phospholipids (in cell memb. ) -cholesterol


And now we will look at: ● NUCLEIC ACIDS & ● PROTEINS

● nucleic acids store and transmit hereditary information ● Two types of nucleic acids: 1) DNA 2) RNA

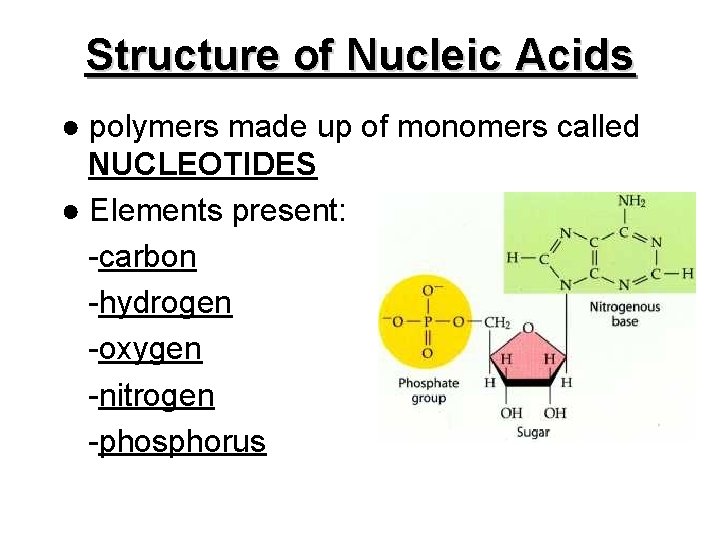
Structure of Nucleic Acids ● polymers made up of monomers called NUCLEOTIDES ● Elements present: -carbon -hydrogen -oxygen -nitrogen -phosphorus

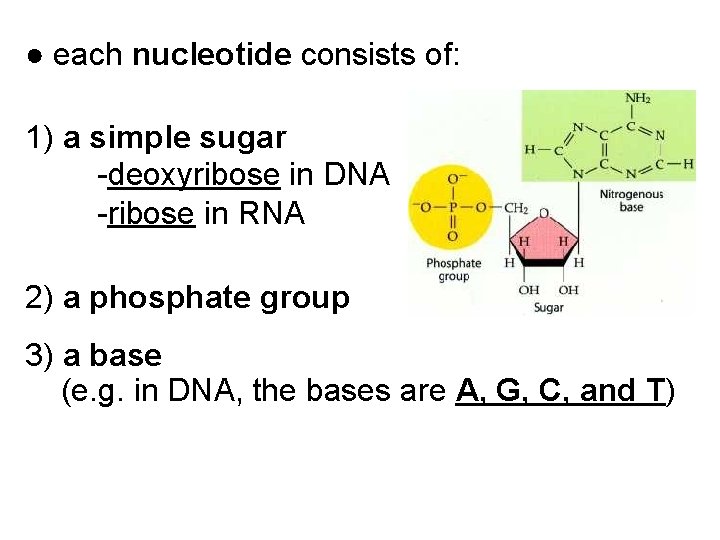
● each nucleotide consists of: 1) a simple sugar -deoxyribose in DNA -ribose in RNA 2) a phosphate group 3) a base (e. g. in DNA, the bases are A, G, C, and T)

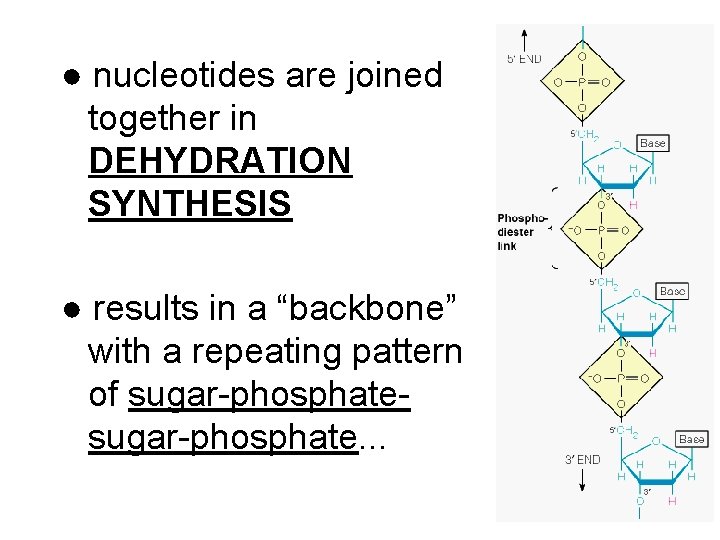
● nucleotides are joined together in DEHYDRATION SYNTHESIS ● results in a “backbone” with a repeating pattern of sugar-phosphate. . .

1) DNA = Deoxyribonucleic acid ● forms the genetic code - the instructions for the proteins (amino acid sequences) of an organisms’ proteins ● is copied and passed from one generation of cells to another

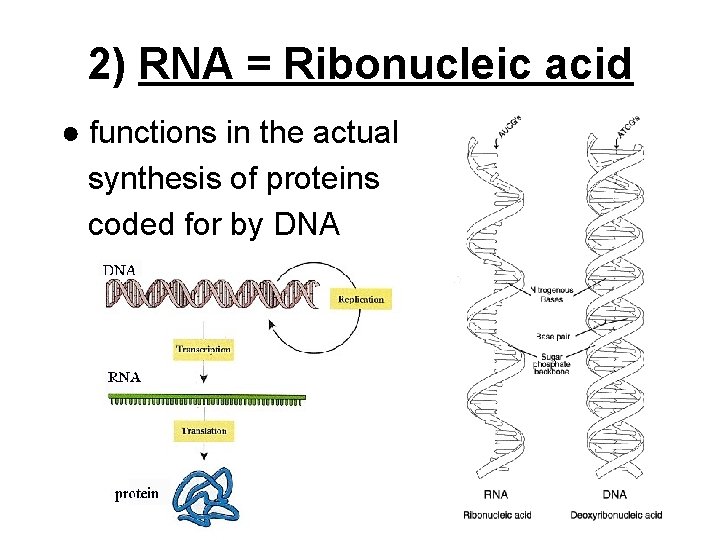
2) RNA = Ribonucleic acid ● functions in the actual synthesis of proteins coded for by DNA

● Polymers (long chains) of AMINO ACIDS – arranged in specific sequence – linked by PEPTIDE BONDS – range in length from a few to 1000+

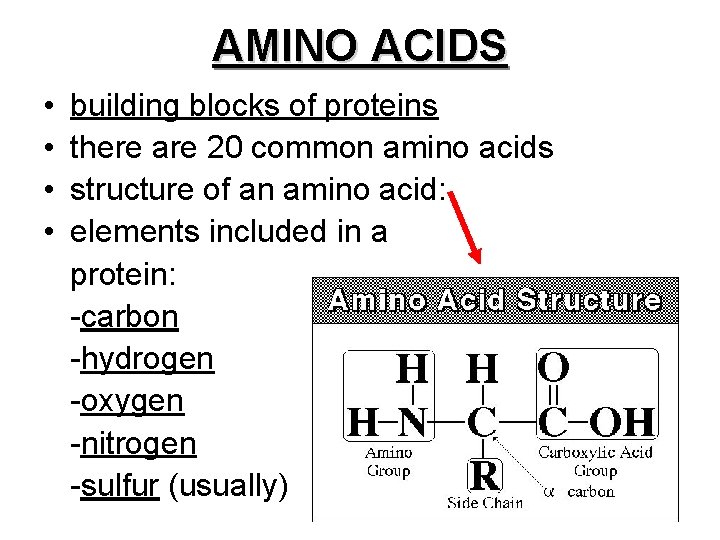
AMINO ACIDS • • building blocks of proteins there are 20 common amino acids structure of an amino acid: elements included in a protein: -carbon -hydrogen -oxygen -nitrogen -sulfur (usually)

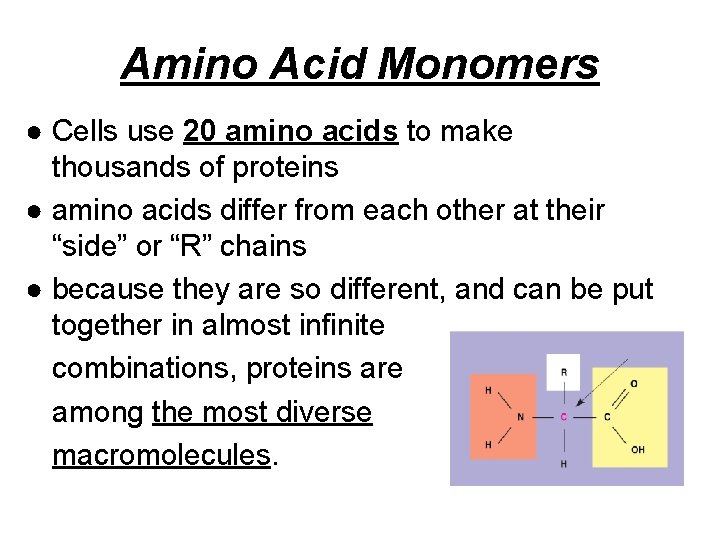
Amino Acid Monomers ● Cells use 20 amino acids to make thousands of proteins ● amino acids differ from each other at their “side” or “R” chains ● because they are so different, and can be put together in almost infinite combinations, proteins are among the most diverse macromolecules.

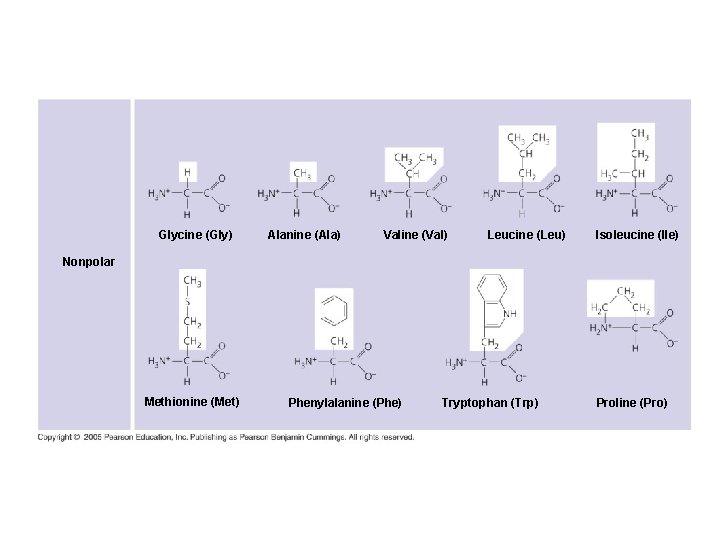
Glycine (Gly) Alanine (Ala) Valine (Val) Leucine (Leu) Isoleucine (Ile) Nonpolar Methionine (Met) Phenylalanine (Phe) Tryptophan (Trp) Proline (Pro)

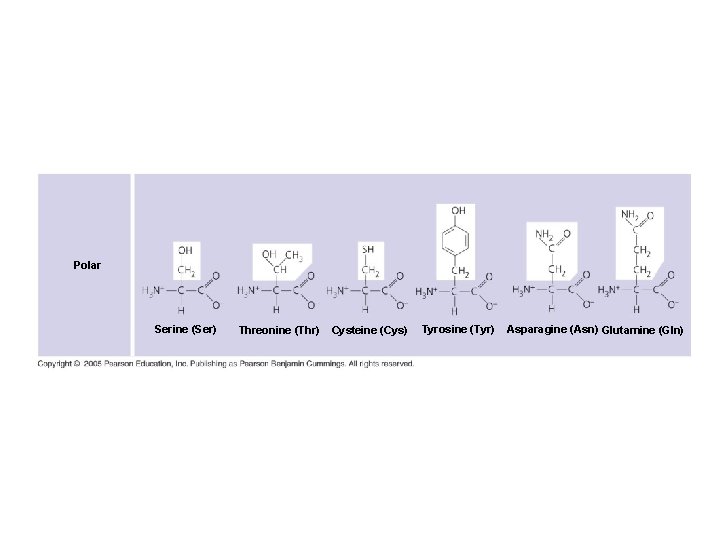
Polar Serine (Ser) Threonine (Thr) Cysteine (Cys) Tyrosine (Tyr) Asparagine (Asn) Glutamine (Gln)

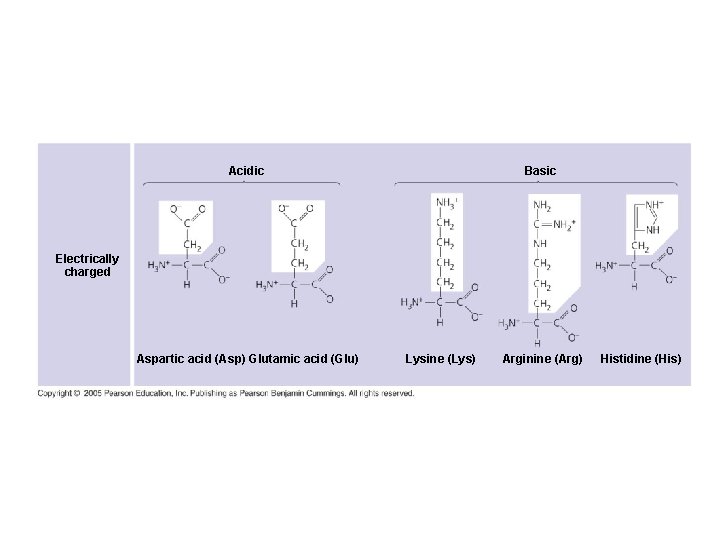
Acidic Basic Electrically charged Aspartic acid (Asp) Glutamic acid (Glu) Lysine (Lys) Arginine (Arg) Histidine (His)

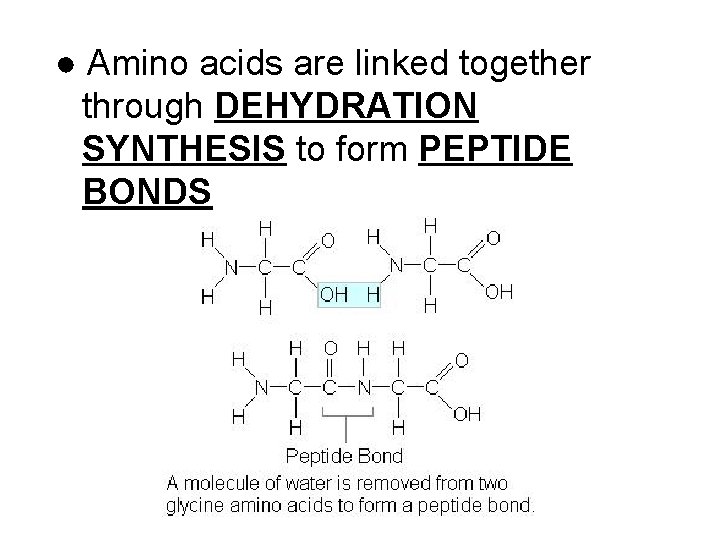
● Amino acids are linked together through DEHYDRATION SYNTHESIS to form PEPTIDE BONDS

PROTEIN STRUCTURE ● a protein’s function depends on its specific 3 -D shape

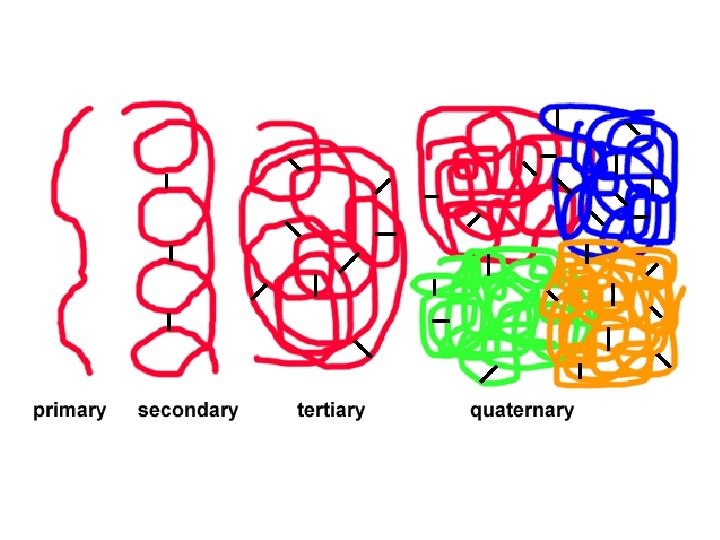
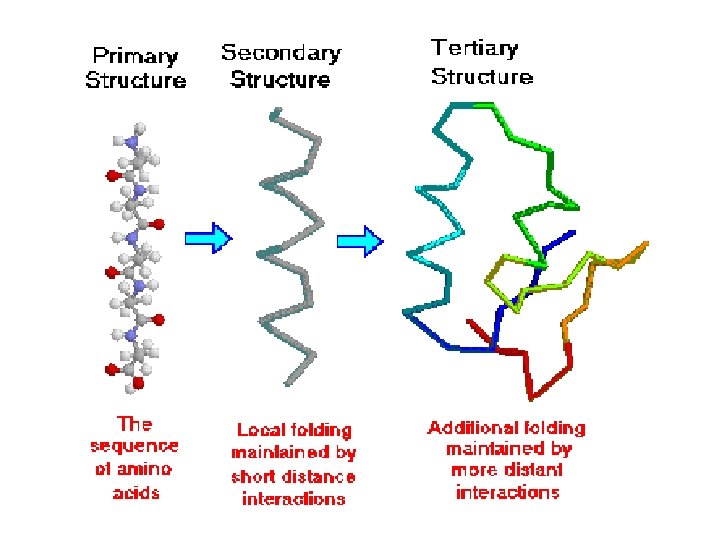
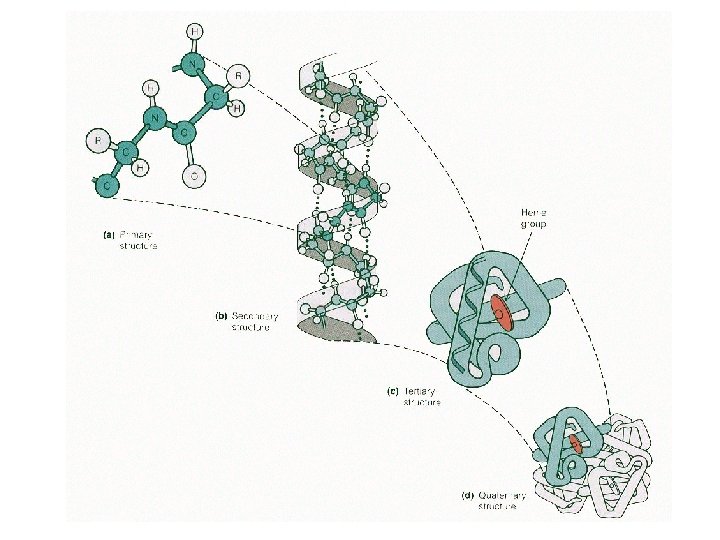
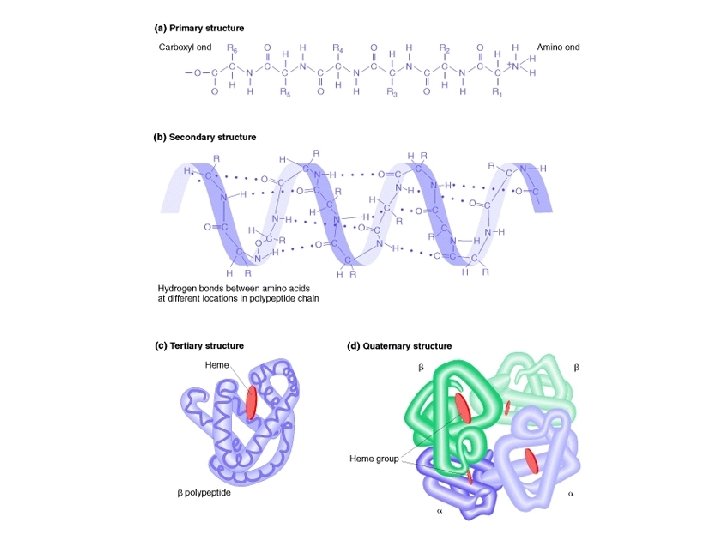
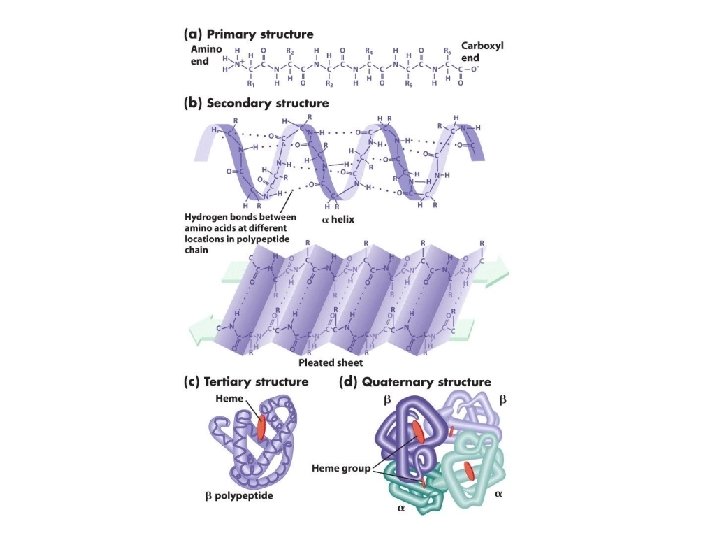
Levels of Protein Shape / Organization: ● amino acids are assembled into polypeptide chains according to instructions coded in DNA! ● there are 4 levels of structure for proteins…

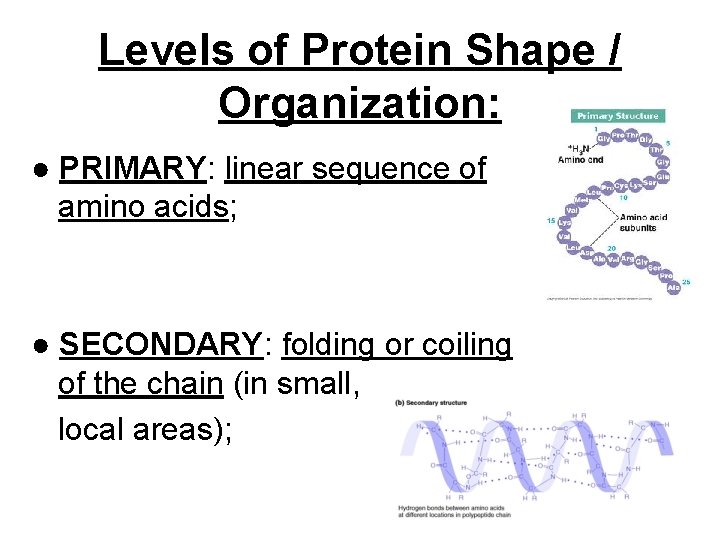
Levels of Protein Shape / Organization: ● PRIMARY: linear sequence of amino acids; ● SECONDARY: folding or coiling of the chain (in small, local areas);

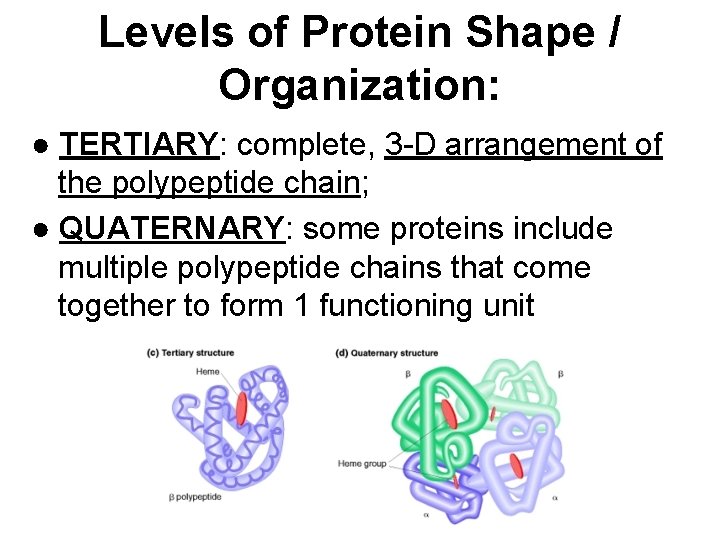
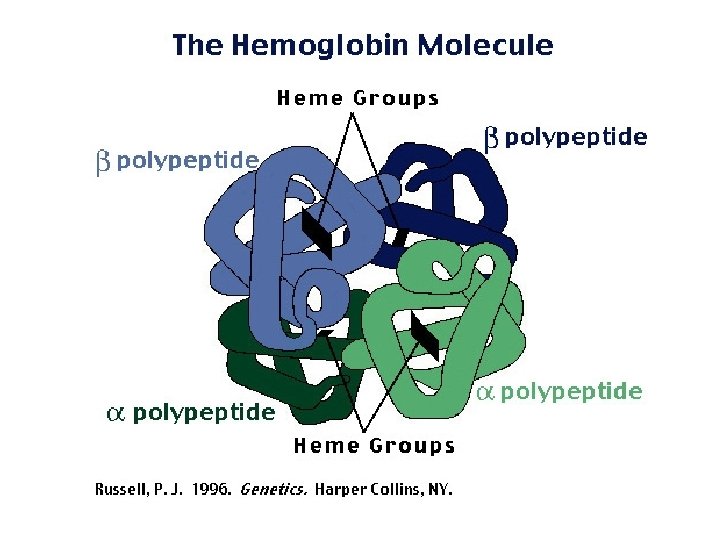
Levels of Protein Shape / Organization: ● TERTIARY: complete, 3 -D arrangement of the polypeptide chain; ● QUATERNARY: some proteins include multiple polypeptide chains that come together to form 1 functioning unit







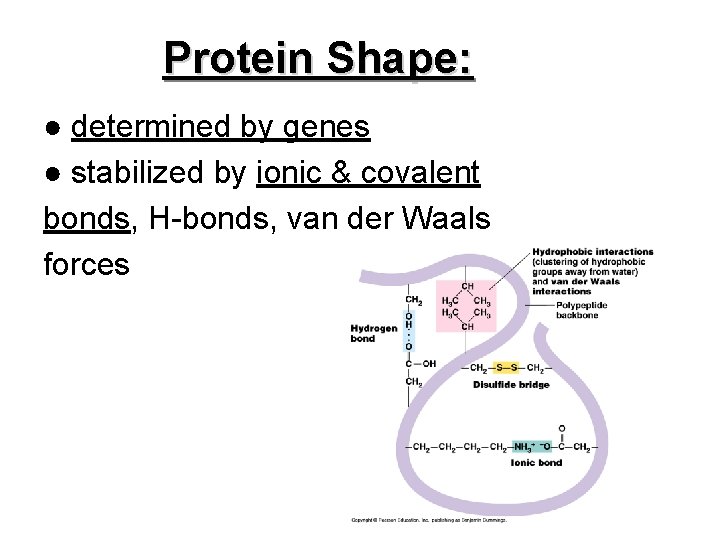
Protein Shape: ● determined by genes ● stabilized by ionic & covalent bonds, H-bonds, van der Waals forces

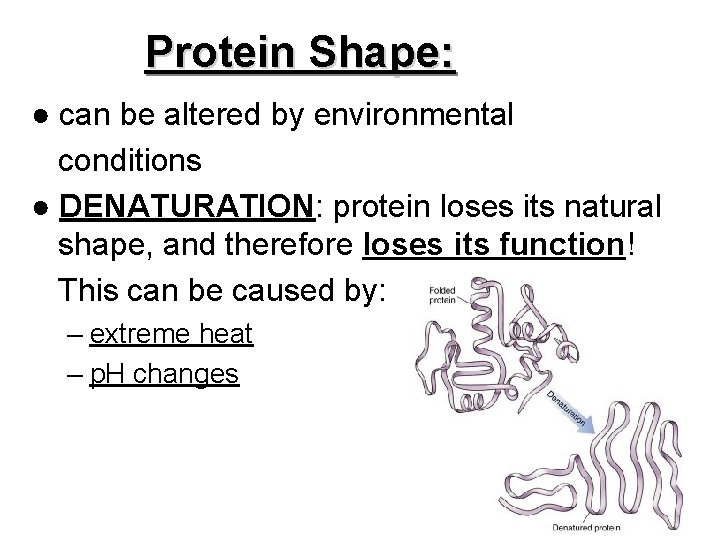
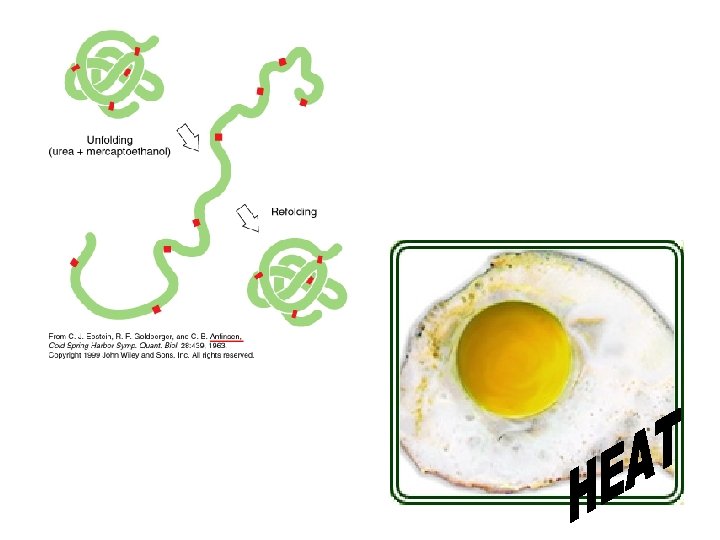
Protein Shape: ● can be altered by environmental conditions ● DENATURATION: protein loses its natural shape, and therefore loses its function! This can be caused by: – extreme heat – p. H changes


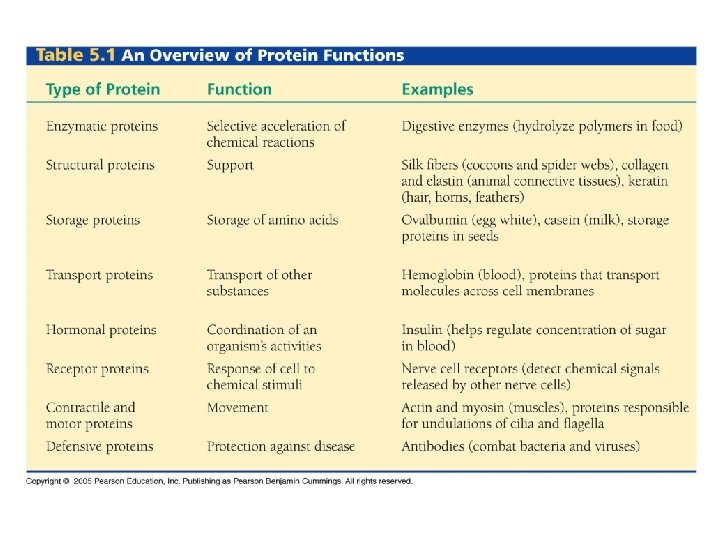
Functions of Proteins ● structural support (e. g. hair, nails) ● signaling (e. g. hormones)

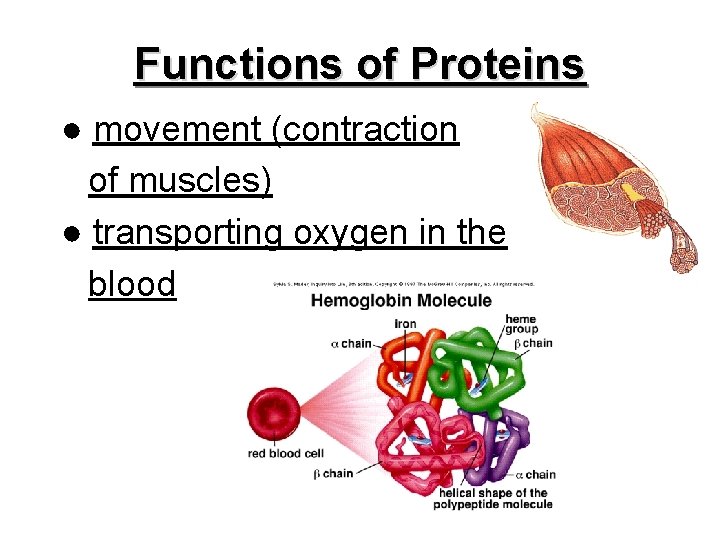
Functions of Proteins ● movement (contraction of muscles) ● transporting oxygen in the blood

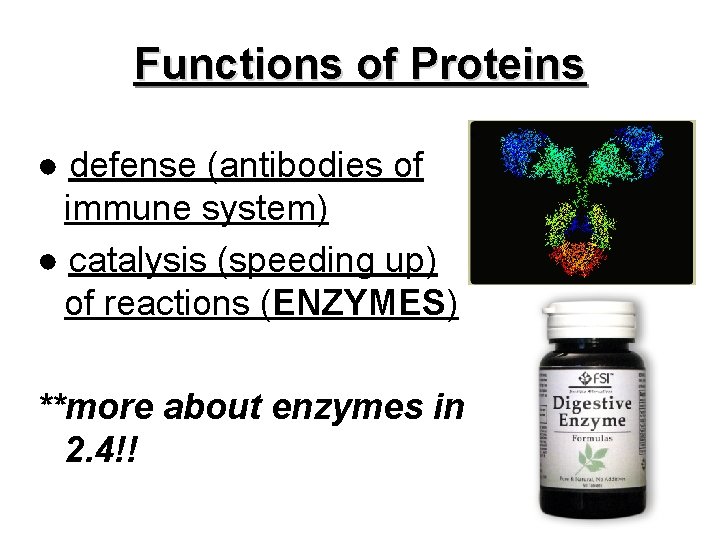
Functions of Proteins ● defense (antibodies of immune system) ● catalysis (speeding up) of reactions (ENZYMES) **more about enzymes in 2. 4!!
