Not happy with your grade Need help understanding
























- Slides: 71

Not happy with your grade? Need help understanding the material? The TLCC Has Free Tutoring

LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 8 An Introduction to Metabolism Lectures by Erin Barley Kathleen Fitzpatrick © 2011 Pearson Education, Inc.

Overview: The Energy of Life • The living cell is a miniature chemical factory where thousands of reactions occur • The cell extracts energy and applies energy to perform work • Some organisms even convert energy to light, as in bioluminescence © 2011 Pearson Education, Inc.

Concept 8. 1: An organism’s metabolism transforms matter and energy, subject to the laws of thermodynamics • Metabolism all of an organism’s chemical reactions • an emergent property of life due to all the interactions between molecules within the cell © 2011 Pearson Education, Inc.

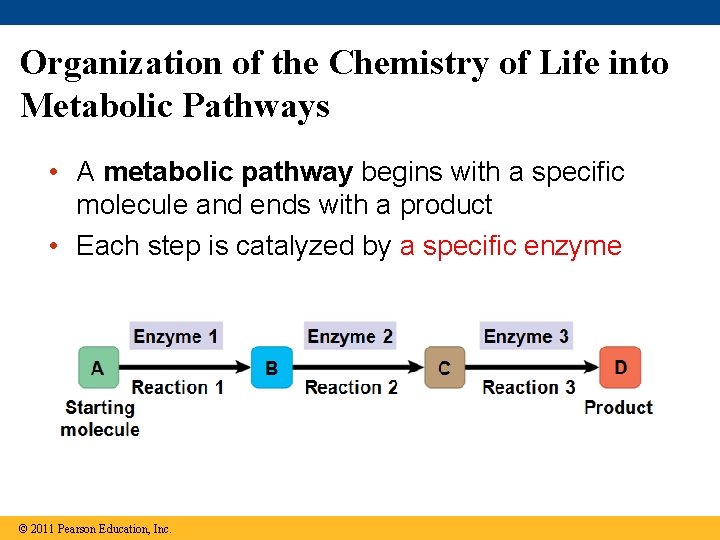
Organization of the Chemistry of Life into Metabolic Pathways • A metabolic pathway begins with a specific molecule and ends with a product • Each step is catalyzed by a specific enzyme © 2011 Pearson Education, Inc.

• Catabolic pathways: release energy by cutting big molecules apart • Anabolic pathways: Add small molecules together to make big ones, needs energy – In many cases, that energy is ATP – Plants use sunlight for energy as they make sugar – Making proteins from amino acids is also anabolic • Cellular respiration, the breakdown of glucose in the presence of oxygen, is an example of a pathway of catabolism © 2011 Pearson Education, Inc.

Forms of Energy • Energy is the capacity to cause change • Energy exists in various forms, some of which can perform work • Bioenergetics is the study of how organisms manage their energy resources © 2011 Pearson Education, Inc.

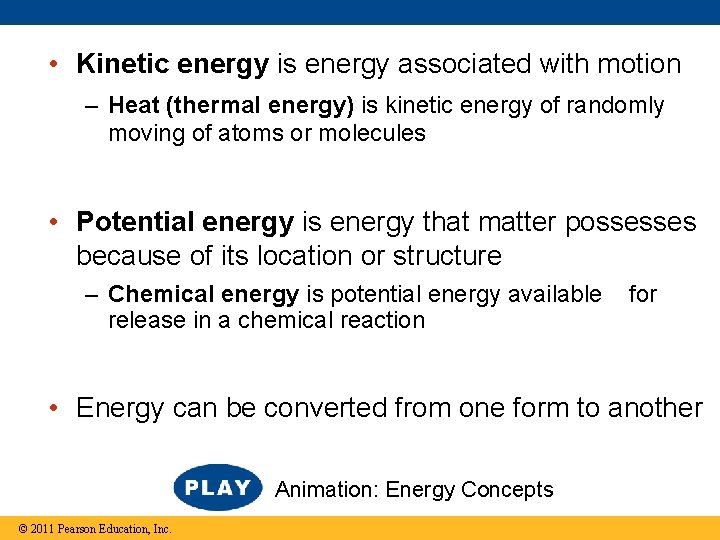
• Kinetic energy is energy associated with motion – Heat (thermal energy) is kinetic energy of randomly moving of atoms or molecules • Potential energy is energy that matter possesses because of its location or structure – Chemical energy is potential energy available release in a chemical reaction for • Energy can be converted from one form to another Animation: Energy Concepts © 2011 Pearson Education, Inc.

Figure 8. 2 A diver has more potential energy on the platform than in the water. Climbing up converts the kinetic energy of muscle movement to potential energy. Diving converts potential energy to kinetic energy. A diver has less potential energy in the water than on the platform.

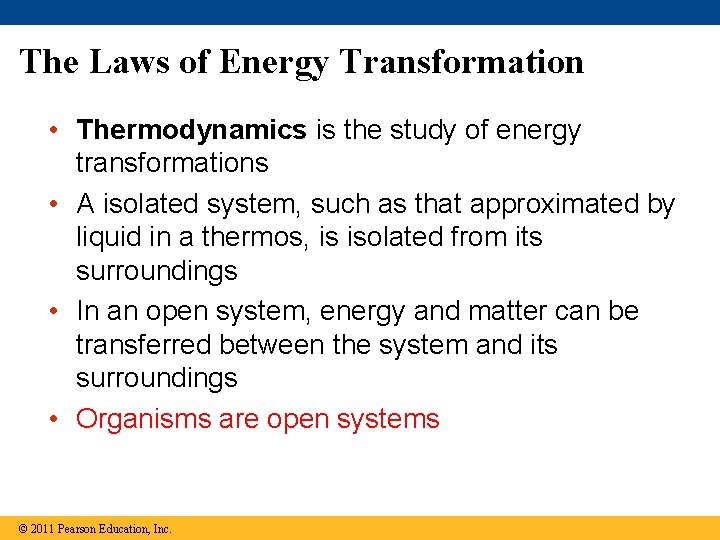
The Laws of Energy Transformation • Thermodynamics is the study of energy transformations • A isolated system, such as that approximated by liquid in a thermos, is isolated from its surroundings • In an open system, energy and matter can be transferred between the system and its surroundings • Organisms are open systems © 2011 Pearson Education, Inc.

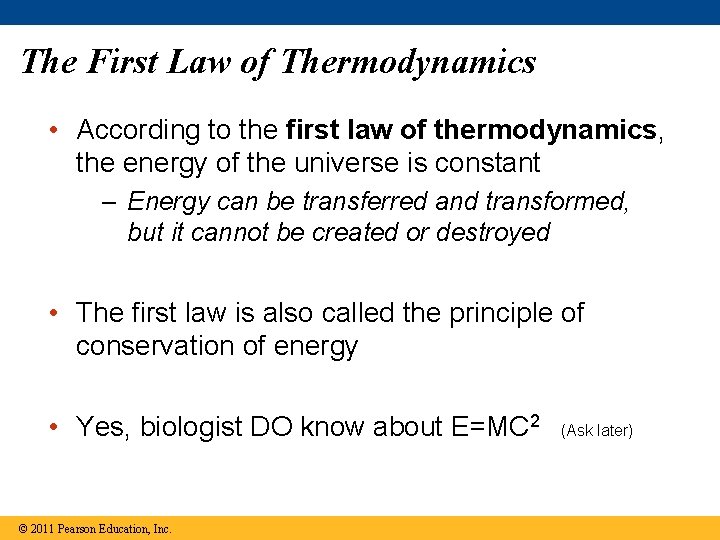
The First Law of Thermodynamics • According to the first law of thermodynamics, the energy of the universe is constant – Energy can be transferred and transformed, but it cannot be created or destroyed • The first law is also called the principle of conservation of energy • Yes, biologist DO know about E=MC 2 © 2011 Pearson Education, Inc. (Ask later)

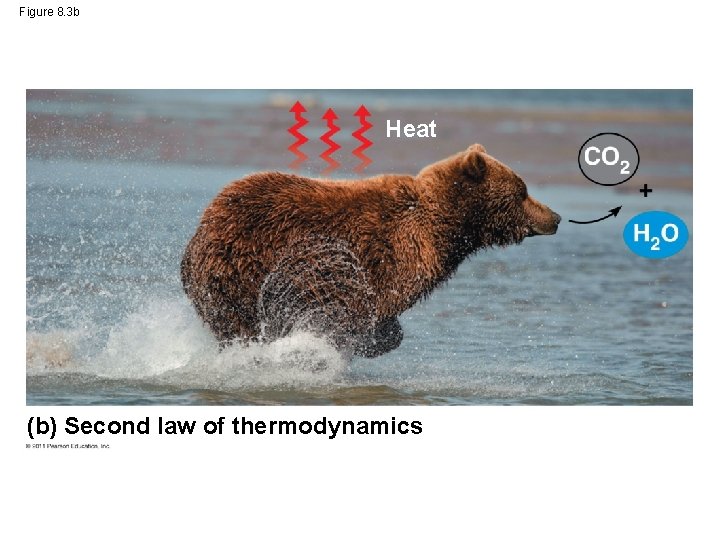
The Second Law of Thermodynamics • Every energy transfer or transformation, wastes some energy in an unusable form (often lost as heat) • According to the second law of thermodynamics – Every energy transfer or transformation increases the entropy (disorder) of the universe (No transfer is 100% efficient) © 2011 Pearson Education, Inc.

Figure 8. 3 a Chemical energy (a) First law of thermodynamics

Figure 8. 3 b Heat (b) Second law of thermodynamics

• Living cells unavoidably convert organized forms of energy (chemical) to heat • Spontaneous processes: no energy used – only happens if it increases the entropy of the universe © 2011 Pearson Education, Inc.

Biological Order and Disorder • Cells: less ordered materials ordered structures • Organisms: ordered matter/energy less ordered • Energy FLOWS THROUGH an ecosystem – Enters as light – exits as heat © 2011 Pearson Education, Inc.

• The evolution of more complex organisms does not violate the second law of thermodynamics • Entropy (disorder) may decrease in an organism, but the universe’s total entropy increases Just like cells don’t violate thermodynamics © 2011 Pearson Education, Inc.

Concept 8. 2: The free-energy change of a reaction tells us whether or not the reaction occurs spontaneously • Biologists want to know which reactions occur spontaneously and which require input of energy • To do so, they need to determine energy changes that occur in chemical reactions © 2011 Pearson Education, Inc.

Free-Energy Change, G • A living system’s free energy is energy that can do work when temperature and pressure are uniform, as in a living cell © 2011 Pearson Education, Inc.

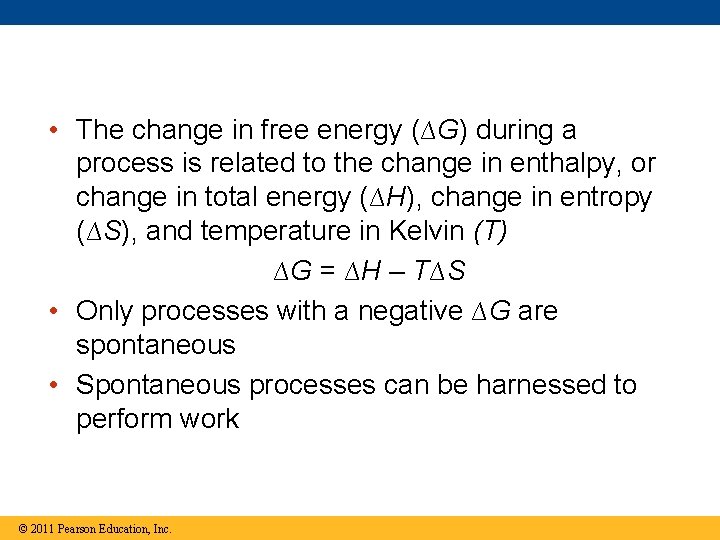
• The change in free energy (∆G) during a process is related to the change in enthalpy, or change in total energy (∆H), change in entropy (∆S), and temperature in Kelvin (T) ∆G = ∆H – T∆S • Only processes with a negative ∆G are spontaneous • Spontaneous processes can be harnessed to perform work © 2011 Pearson Education, Inc.

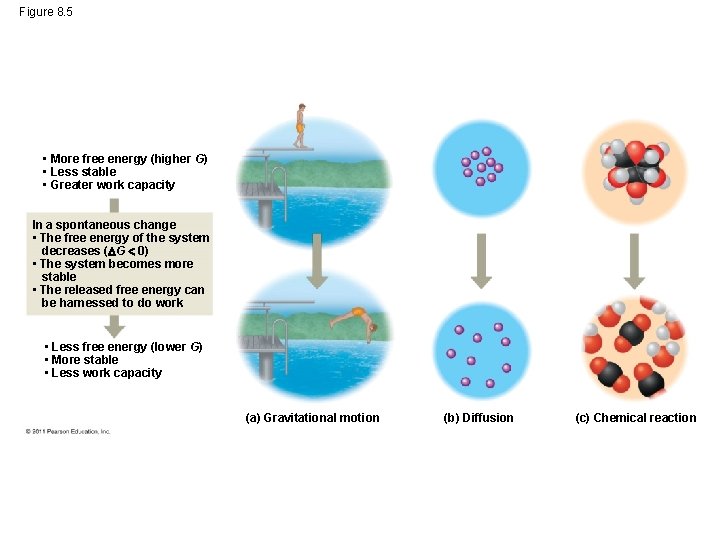
Free Energy, Stability, and Equilibrium • Free energy is a measure of a system’s instability, its tendency to change to a more stable state • During a spontaneous change, free energy decreases and the stability of a system increases • Equilibrium is a state of maximum stability • A process is spontaneous and can perform work only when it is moving toward equilibrium © 2011 Pearson Education, Inc.

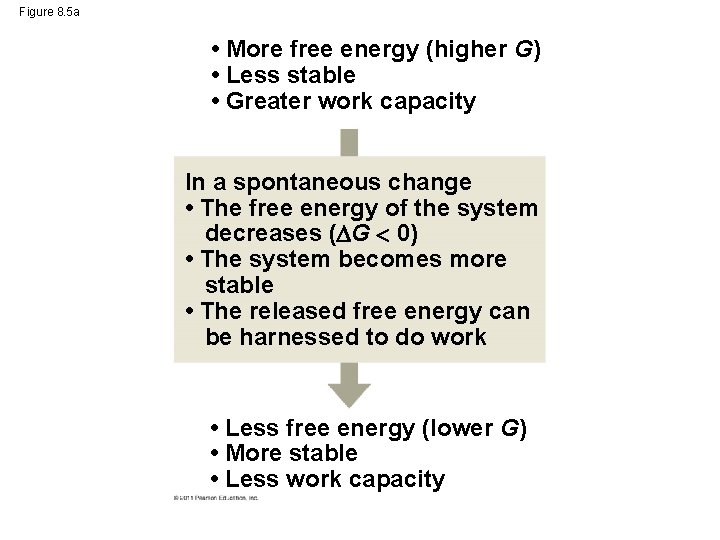
Figure 8. 5 • More free energy (higher G) • Less stable • Greater work capacity In a spontaneous change • The free energy of the system decreases ( G 0) • The system becomes more stable • The released free energy can be harnessed to do work • Less free energy (lower G) • More stable • Less work capacity (a) Gravitational motion (b) Diffusion (c) Chemical reaction

Figure 8. 5 a • More free energy (higher G) • Less stable • Greater work capacity In a spontaneous change • The free energy of the system decreases ( G 0) • The system becomes more stable • The released free energy can be harnessed to do work • Less free energy (lower G) • More stable • Less work capacity

Figure 8. 5 b (a) Gravitational motion (b) Diffusion (c) Chemical reaction

Free Energy and Metabolism • The concept of free energy can be applied to the chemistry of life’s processes © 2011 Pearson Education, Inc.

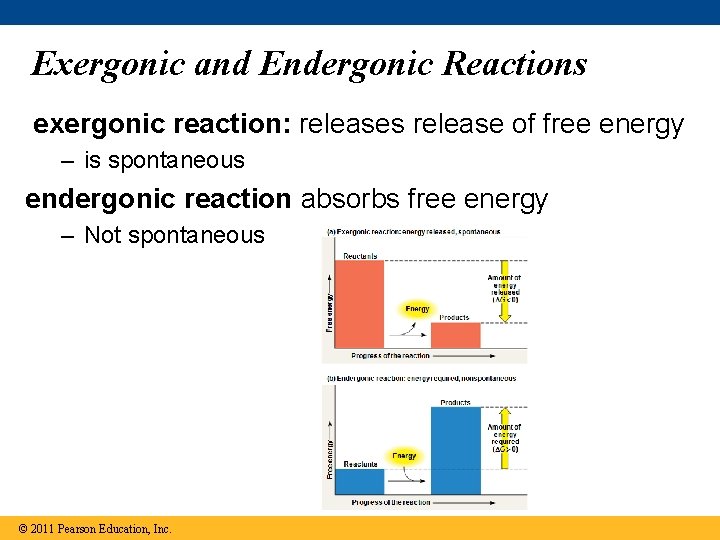
Exergonic and Endergonic Reactions exergonic reaction: releases release of free energy – is spontaneous endergonic reaction absorbs free energy – Not spontaneous © 2011 Pearson Education, Inc.

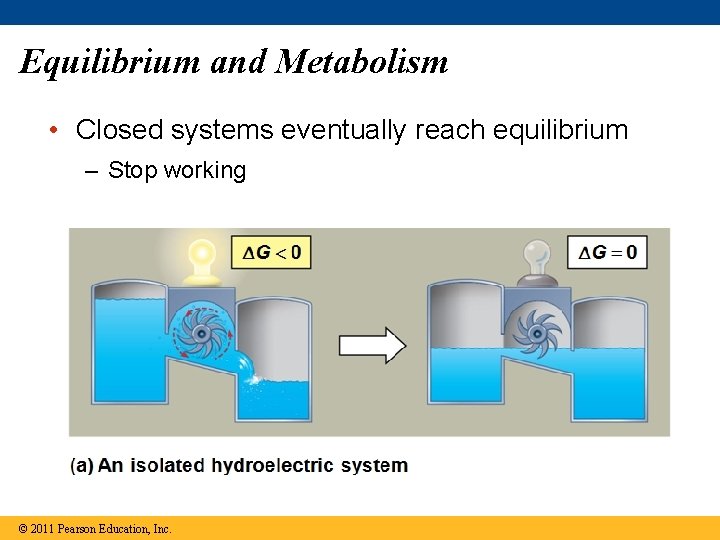
Equilibrium and Metabolism • Closed systems eventually reach equilibrium – Stop working © 2011 Pearson Education, Inc.

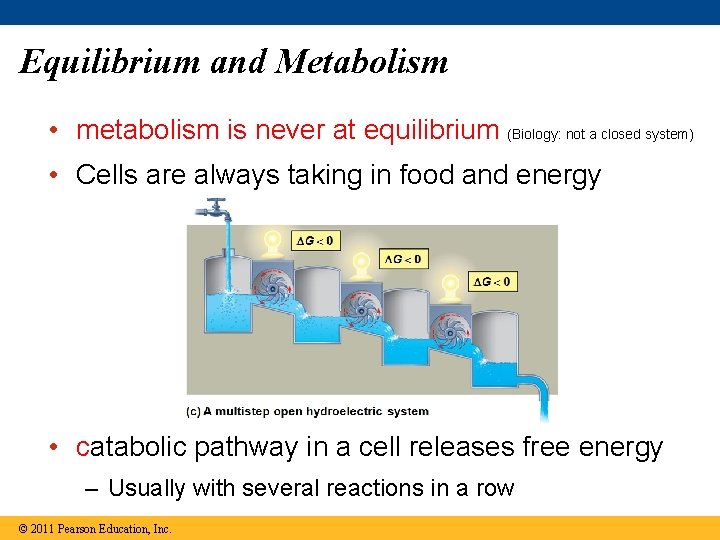
Equilibrium and Metabolism • metabolism is never at equilibrium (Biology: not a closed system) • Cells are always taking in food and energy • catabolic pathway in a cell releases free energy – Usually with several reactions in a row © 2011 Pearson Education, Inc.

Concept 8. 3: ATP powers cellular work • A cell does three main kinds of work – Chemical – Transport – Mechanical • energy coupling (exergonic + endergonic) – Exergonic: breaking energy rich molecules (usually ATP) – use that energy to power endergonic reactions • ATP is the main fuel to power the cell © 2011 Pearson Education, Inc.

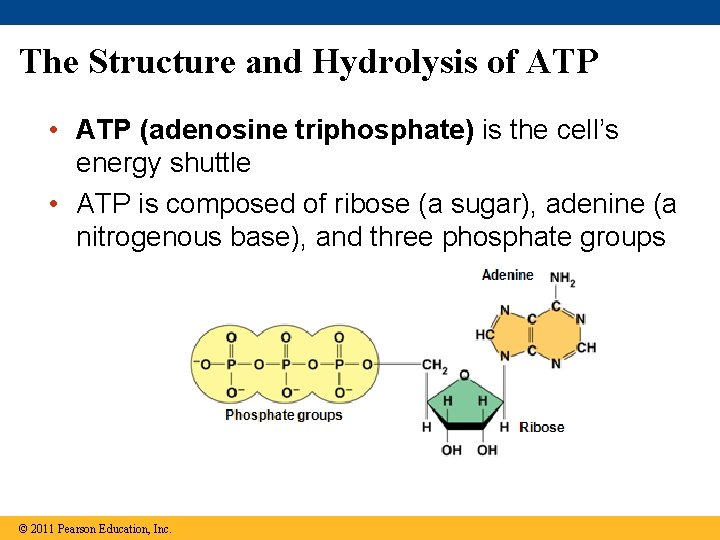
The Structure and Hydrolysis of ATP • ATP (adenosine triphosphate) is the cell’s energy shuttle • ATP is composed of ribose (a sugar), adenine (a nitrogenous base), and three phosphate groups © 2011 Pearson Education, Inc.

• The bonds between the phosphate groups of ATP’s tail can be broken by hydrolysis • Energy is released from ATP when the terminal phosphate bond is broken © 2011 Pearson Education, Inc.

How the Hydrolysis of ATP Performs Work • All three types of cellular work (mechanical, transport, and chemical) are powered by the hydrolysis of ATP • Breaking ATP is exergonic – The energy released is used to drive endergonic reactions • Overall, the coupled reactions are exergonic – More energy is released than is used © 2011 Pearson Education, Inc.

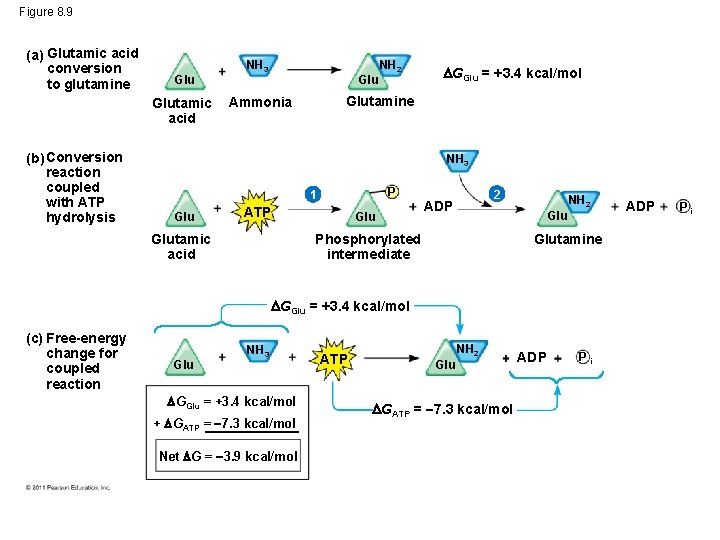
Figure 8. 9 (a) Glutamic acid conversion to glutamine Glutamic acid (b) Conversion reaction coupled with ATP hydrolysis NH 3 Glu NH 2 GGlu = +3. 4 kcal/mol Glutamine Ammonia NH 3 P 1 Glu ATP Glu 2 ADP Glu Phosphorylated intermediate Glutamic acid NH 2 Glutamine GGlu = +3. 4 kcal/mol (c) Free-energy change for coupled reaction Glu NH 3 GGlu = +3. 4 kcal/mol + GATP = 7. 3 kcal/mol Net G = 3. 9 kcal/mol ATP NH 2 Glu GATP = 7. 3 kcal/mol ADP Pi

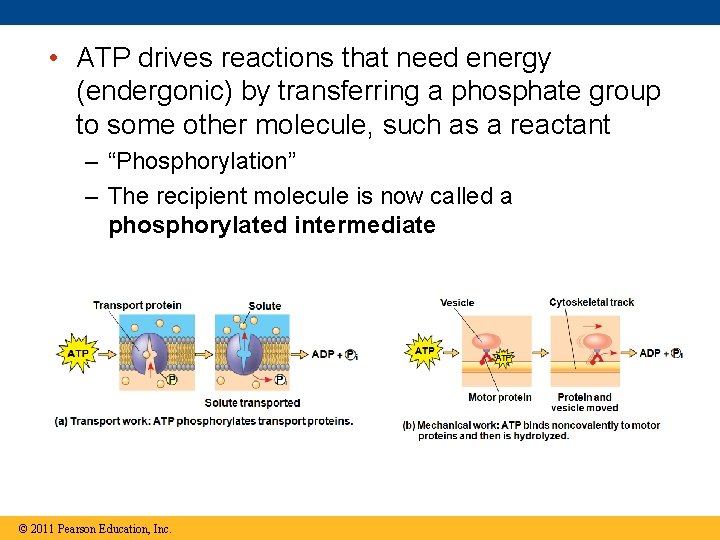
• ATP drives reactions that need energy (endergonic) by transferring a phosphate group to some other molecule, such as a reactant – “Phosphorylation” – The recipient molecule is now called a phosphorylated intermediate © 2011 Pearson Education, Inc.

The Regeneration of ATP • ATP is a renewable resource – ADP + P • Uses energy from catabolic reactions • Glucose is broken down to make ATP, which powers other reactions • Coal is burned in power plant to make electricity, which powers lights, stove, etc. • Difference: ATD and P can be used again and again © 2011 Pearson Education, Inc.

Figure 8. 11 ATP Energy from catabolism (exergonic, energy-releasing processes) ADP H 2 O Pi Energy for cellular work (endergonic, energy-consuming processes)

Concept 8. 4: Enzymes speed up metabolic reactions by lowering energy barriers • Catalyst: speeds up chemical reaction without being used up in reaction • An enzyme is a catalytic protein • Hydrolysis of sucrose by the enzyme sucrase is an example of an enzyme-catalyzed reaction © 2011 Pearson Education, Inc.

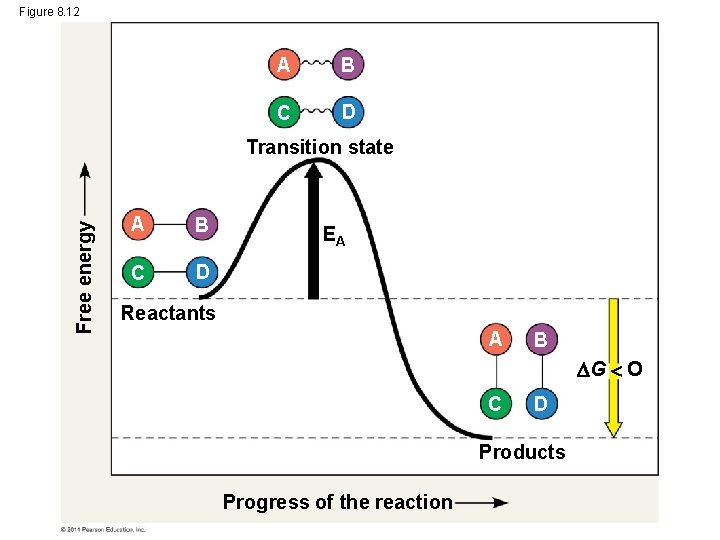
The Activation Energy Barrier • chemical reaction: breaking and making bonds • activation energy (EA) The initial energy needed to start a chemical reaction • Activation energy is often supplied in the form of thermal energy that the reactant molecules absorb from their surroundings © 2011 Pearson Education, Inc.

Figure 8. 12 A B C D Free energy Transition state A B C D EA Reactants A B G O C D Products Progress of the reaction

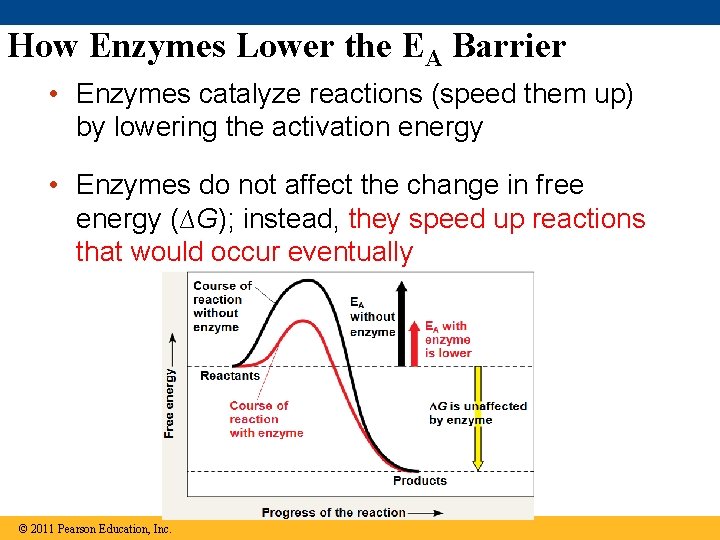
How Enzymes Lower the EA Barrier • Enzymes catalyze reactions (speed them up) by lowering the activation energy • Enzymes do not affect the change in free energy (∆G); instead, they speed up reactions that would occur eventually Animation: How Enzymes © 2011 Pearson Education, Inc.

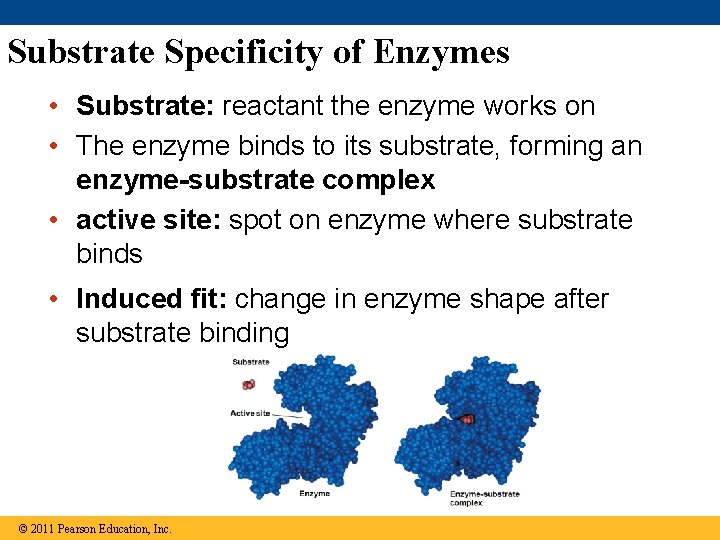
Substrate Specificity of Enzymes • Substrate: reactant the enzyme works on • The enzyme binds to its substrate, forming an enzyme-substrate complex • active site: spot on enzyme where substrate binds • Induced fit: change in enzyme shape after substrate binding © 2011 Pearson Education, Inc.

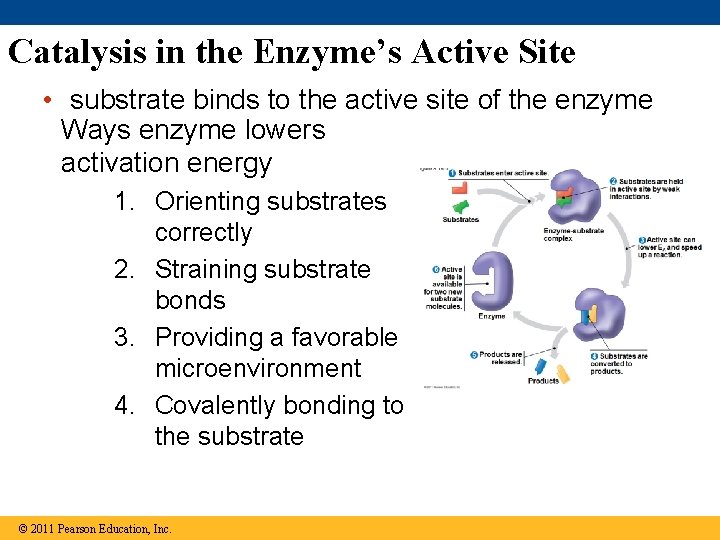
Catalysis in the Enzyme’s Active Site • substrate binds to the active site of the enzyme Ways enzyme lowers activation energy 1. Orienting substrates correctly 2. Straining substrate bonds 3. Providing a favorable microenvironment 4. Covalently bonding to the substrate © 2011 Pearson Education, Inc.

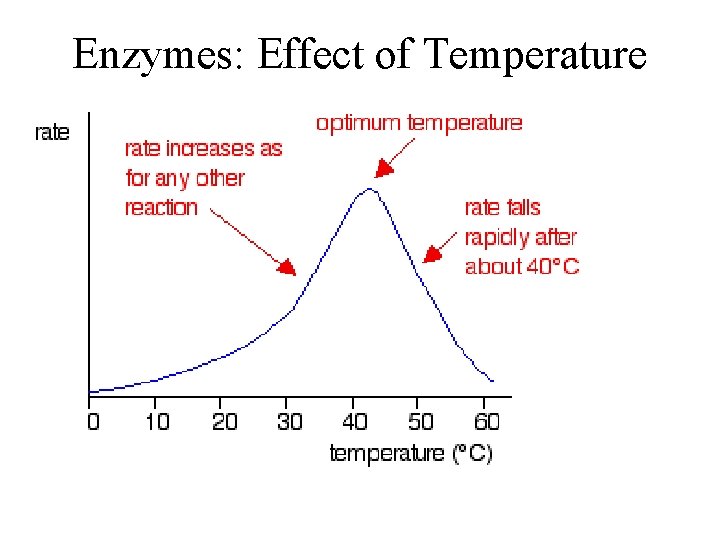
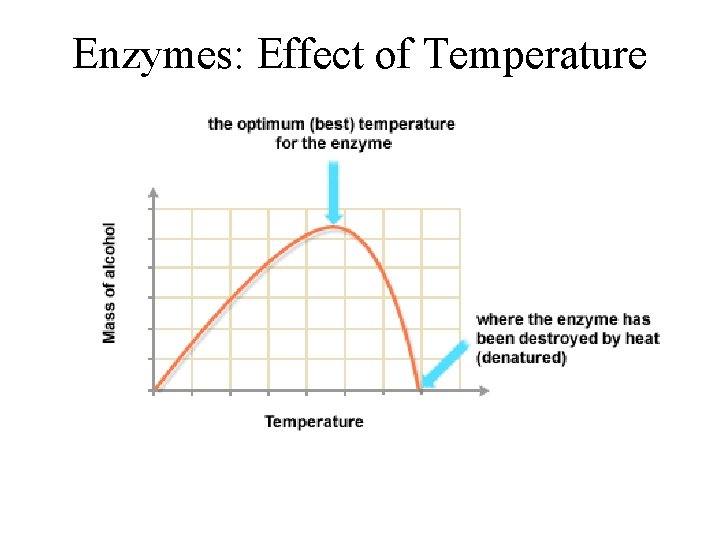
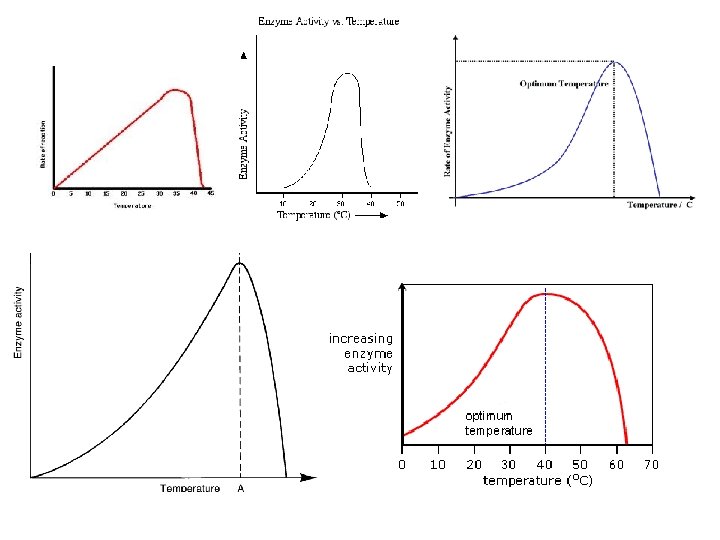
Conditions that affect Enzyme Activity • An enzyme’s activity can be affected by – General environmental factors, such as temperature and p. H • If you denature it, you stop the reactions • If you slow down the collisions, you reduce the reactions – Chemicals that specifically influence the enzyme • Inhibitor molecules © 2011 Pearson Education, Inc.

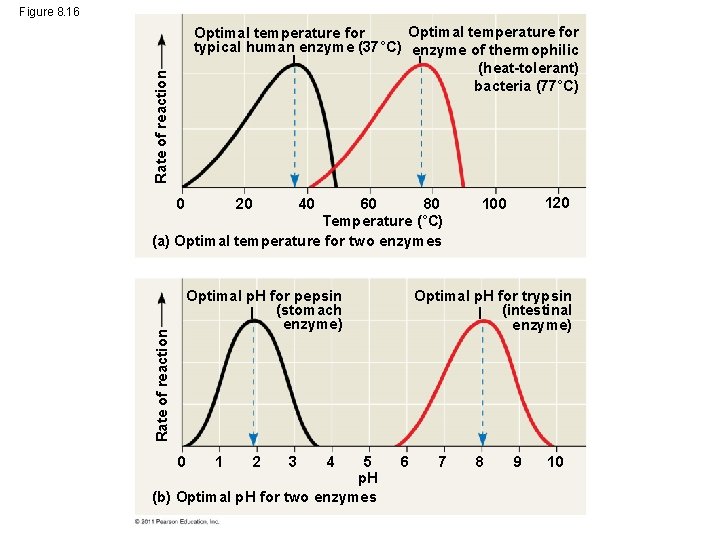
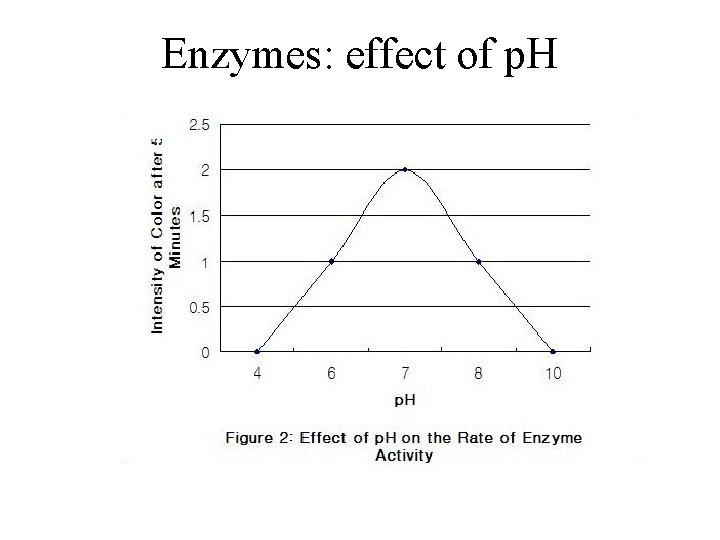
Effects of Temperature and p. H • Each enzyme has an optimal temperature in which it can function • Each enzyme has an optimal p. H in which it can function • Optimal conditions favor the most active shape for the enzyme molecule © 2011 Pearson Education, Inc.

Figure 8. 16 Rate of reaction Optimal temperature for typical human enzyme (37°C) enzyme of thermophilic (heat-tolerant) bacteria (77°C) 60 80 Temperature (°C) (a) Optimal temperature for two enzymes 0 20 40 Rate of reaction Optimal p. H for pepsin (stomach enzyme) 0 5 p. H (b) Optimal p. H for two enzymes 1 2 3 4 120 100 Optimal p. H for trypsin (intestinal enzyme) 6 7 8 9 10

Cofactors • Cofactors: non-protein enzyme helpers – Can be inorganic (e. g. a metal ion) or organic • An organic cofactor is called a coenzyme • Coenzymes include vitamins © 2011 Pearson Education, Inc.

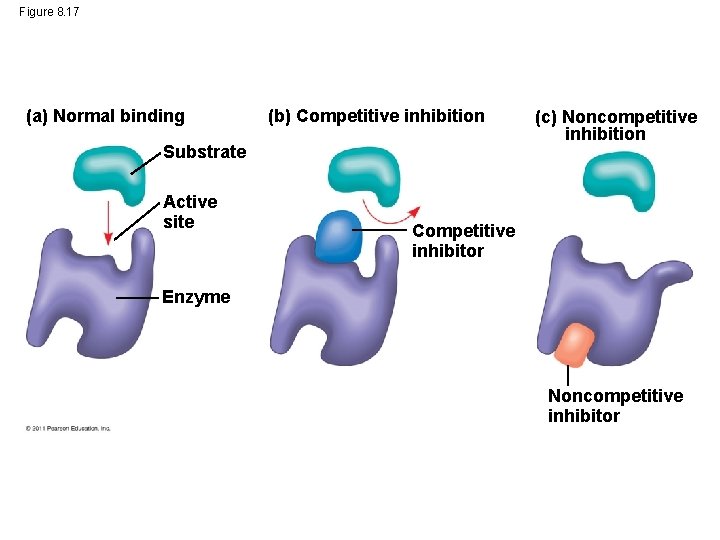
Enzyme Inhibitors • Competitive inhibitors: bind to the active site of an enzyme, competing with the substrate • Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective (“allosteric inhibitor”) • Examples of inhibitors include toxins, poisons, pesticides, and antibiotics © 2011 Pearson Education, Inc.

Figure 8. 17 (a) Normal binding (b) Competitive inhibition Substrate Active site (c) Noncompetitive inhibition Competitive inhibitor Enzyme Noncompetitive inhibitor

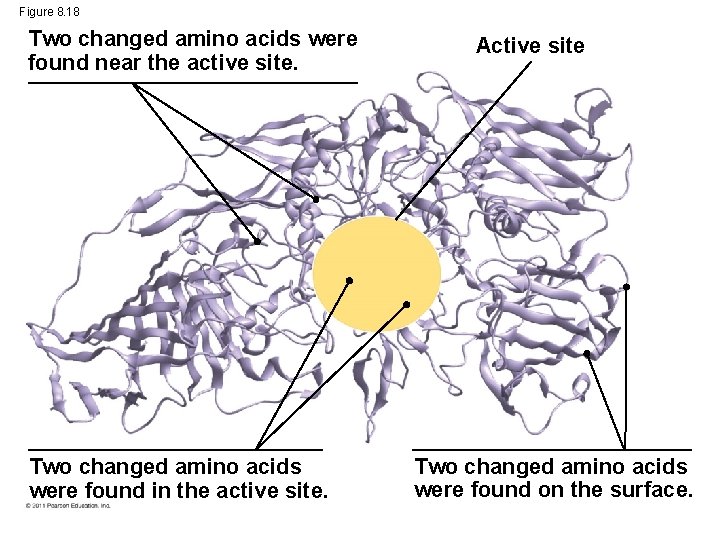
The Evolution of Enzymes • Enzymes are proteins encoded by genes • Changes (mutations) in genes lead to changes in amino acid composition of an enzyme • Altered amino acids in enzymes may alter their substrate specificity • Under new environmental conditions a novel form of an enzyme might be favored © 2011 Pearson Education, Inc.

Figure 8. 18 Two changed amino acids were found near the active site. Two changed amino acids were found in the active site. Active site Two changed amino acids were found on the surface.

Concept 8. 5: Regulation of enzyme activity helps control metabolism • metabolic pathways must be tightly regulated • Two methods a cell uses 1. Starting or stopping production of an enzyme • A cell does this by switching on or off the genes with instructions to make that specific enzyme 2. regulating the activity of existing enzymes © 2011 Pearson Education, Inc.

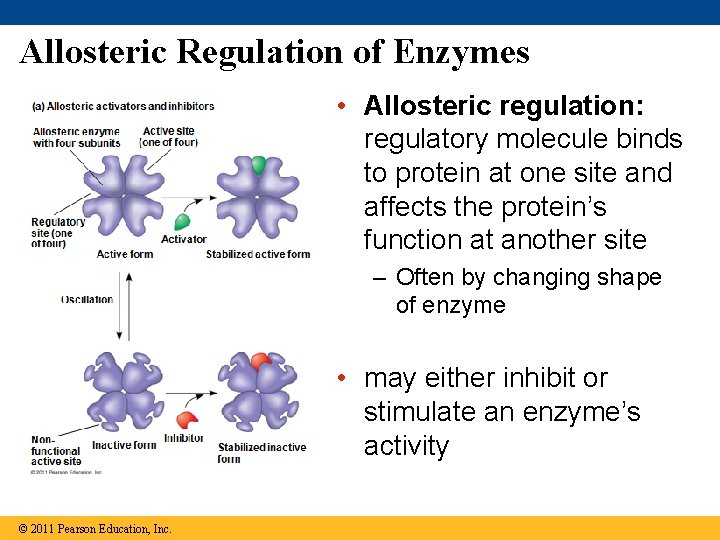
Allosteric Regulation of Enzymes • Allosteric regulation: regulatory molecule binds to protein at one site and affects the protein’s function at another site – Often by changing shape of enzyme • may either inhibit or stimulate an enzyme’s activity © 2011 Pearson Education, Inc.

Allosteric Activation and Inhibition • Most allosterically regulated enzymes are made from several polypeptide subunits – have active and inactive forms – Binding to activator molecule stabilizes the active form of the enzyme – Binding to an inhibitor molecule stabilizes the inactive form of the enzyme © 2011 Pearson Education, Inc.

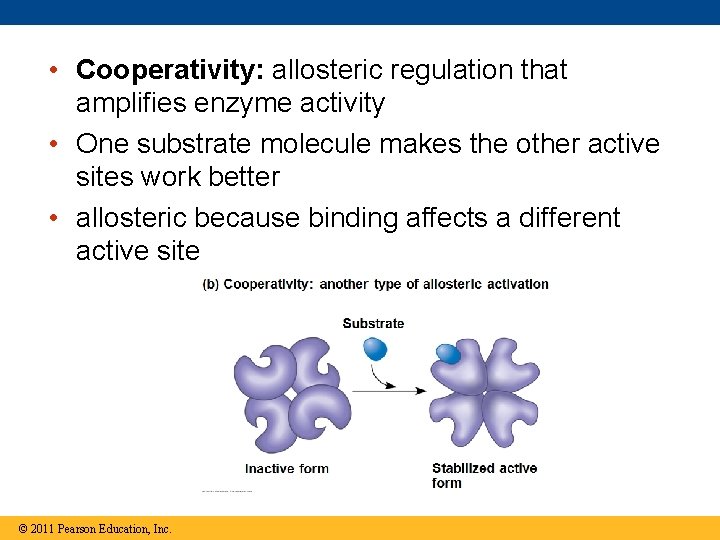
• Cooperativity: allosteric regulation that amplifies enzyme activity • One substrate molecule makes the other active sites work better • allosteric because binding affects a different active site © 2011 Pearson Education, Inc.

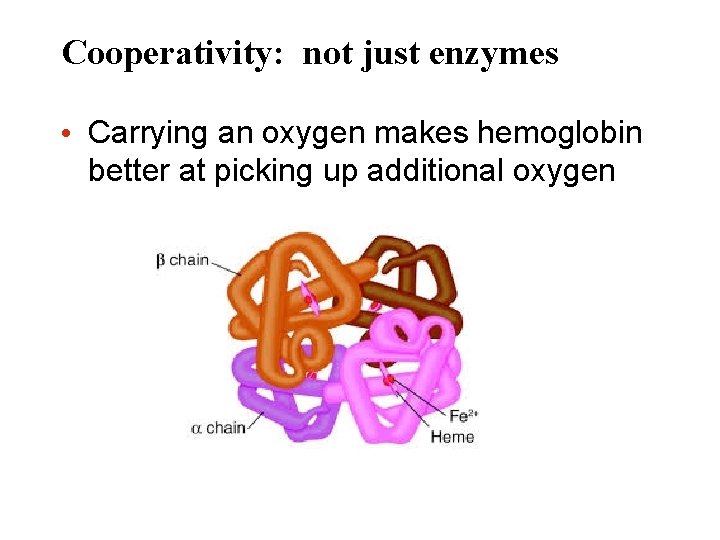
Cooperativity: not just enzymes • Carrying an oxygen makes hemoglobin better at picking up additional oxygen

Identification of Allosteric Regulators • Allosteric regulators are attractive drug candidates for enzyme regulation because of their specificity • Inhibition of proteolytic enzymes called caspases may help management of inappropriate inflammatory responses © 2011 Pearson Education, Inc.

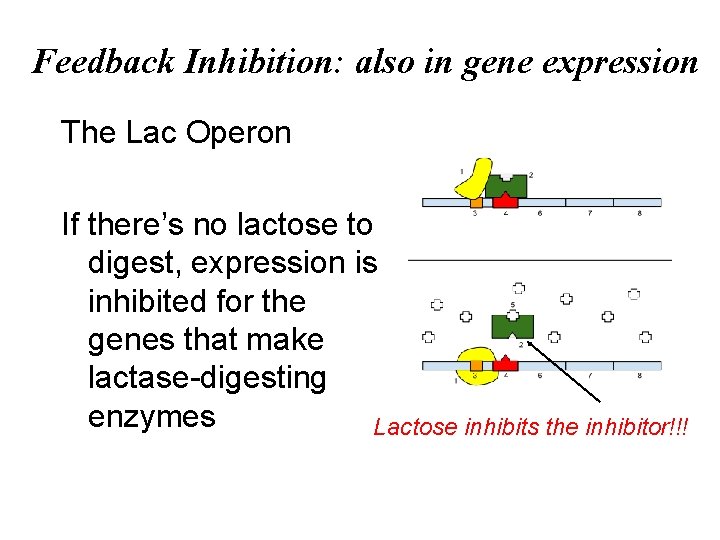
Feedback Inhibition • In feedback inhibition, the end product of a metabolic pathway shuts down the pathway • Feedback inhibition prevents a cell from wasting chemical resources by synthesizing more product than is needed © 2011 Pearson Education, Inc.

Feedback Inhibition: also in gene expression The Lac Operon If there’s no lactose to digest, expression is inhibited for the genes that make lactase-digesting enzymes Lactose inhibits the inhibitor!!!

Specific Localization of Enzymes Within the Cell • metabolic pathways work better because of structures within the cell – Some enzymes act as structural components of membranes – In eukaryotic cells, some enzymes reside in specific organelles; for example, enzymes for cellular respiration are located in mitochondria © 2011 Pearson Education, Inc.

Enzyme Localization in mitochondria – enzymes for cellular respiration are located in mitochondria © 2011 Pearson Education, Inc.

Concept Quiz Why are high fevers dangerous and sometimes life‑threatening? A. Molecules move faster at higher temperatures. B. Enzymes may change shape at high temperatures. C. Invading microbes survive better and reproduce faster at high temperatures.

Concept Quiz Where a substrate binds to an enzyme is known as the A. Active site B. Activation energy C. Energy transfer site

Enzymes: Effect of Temperature

Enzymes: Effect of Temperature


Enzymes: effect of p. H


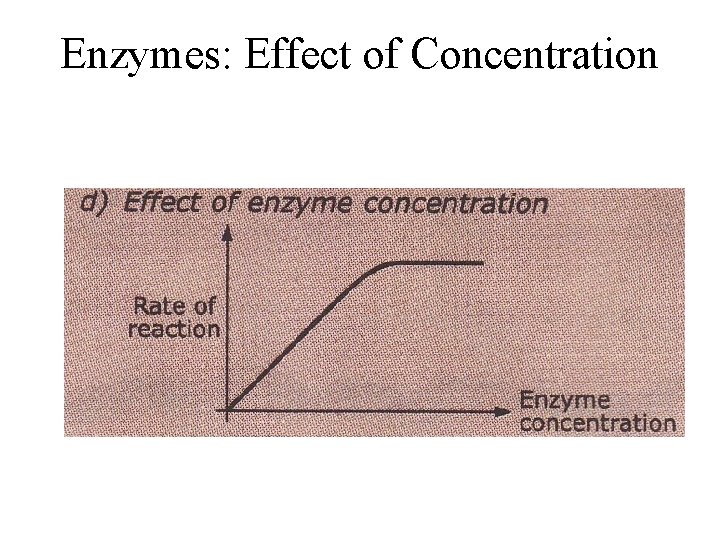
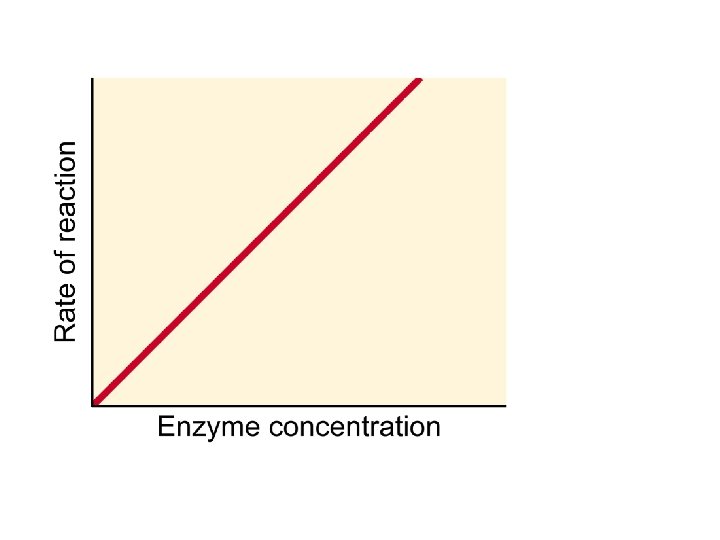
Enzymes: Effect of Concentration



Not happy with your grade? Need help understanding the material? The TLCC Has Free Tutoring