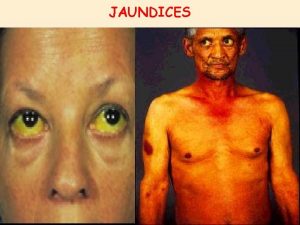
NOON REPORT CC JAUNDICE Focus diagnostics complications of






















- Slides: 35

NOON REPORT CC: JAUNDICE Focus: diagnostics, complications of transplant Anna Stecher 4/29/2020 Chief facilitator: Riana Wurzburger

HPI: • 41 year old female with cystic fibrosis c/b cirrhosis further c/b HRS, now s/p liver and kidney transplant in 2014, on immunosuppression with mycophenolate, prednisone, and tacrolimus How common is cirrhosis in CF? • ~40 percent of individuals with CF develop • Main complaints are: clinically detectable CF-related liver disease • Jaundice (CFLD) • ~ 20 percent of these (5 to 10 percent of individuals • Abdominal pain with CF) go on to develop cirrhosis • Decreased appetite • Pathogenesis? Multiple mechanisms, but a big factor is the dysfunctional CFTR, which leads to • All occurring over past 5 -7 days thick bile that congests intrahepatic bile ducts over • No pulmonary complaints time Leung D & Narkewicz M. Cystic fibrosis: Hepatobiliary disease. In: Up. To. Date, Post TW (Ed), Up. To. Date, Waltham, MA. (Accessed on April 29, 2020. )

HPI/ROS: • Constitutional: + Mild fatigue; no weight change, f/c, night sweats • Cardiovascular: No chest pain, palpitations • Respiratory: No cough, no sputum changes, no dyspnea, no changes to CF regimen • Gastrointestinal: + Nausea, small emesis, mild diffuse abdominal pain • Skin: + Jaundice • Neuro: Noncontributory • Heme/Lymph: No bruising, bleeding, lymphadenopathy

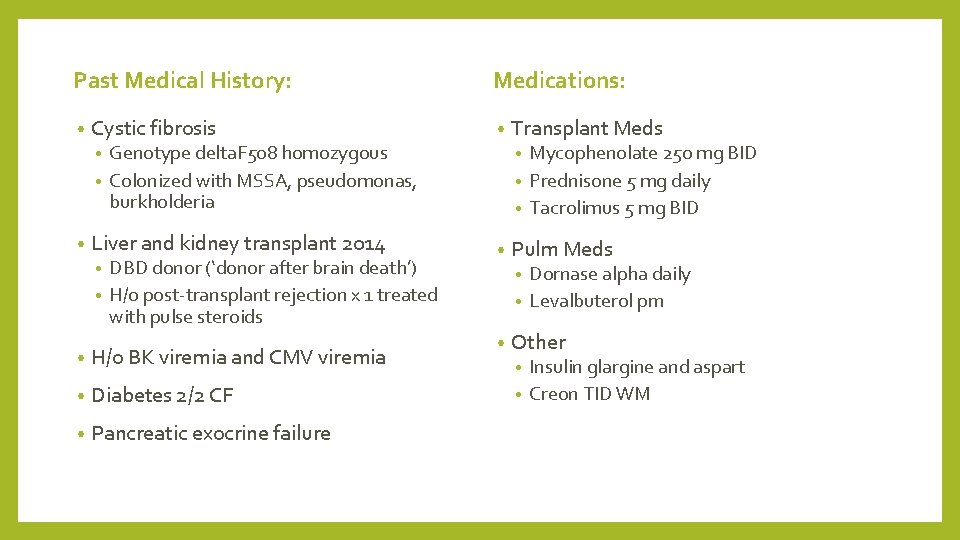
Past Medical History: • Cystic fibrosis Medications: • Mycophenolate 250 mg BID • Prednisone 5 mg daily • Tacrolimus 5 mg BID Genotype delta. F 508 homozygous • Colonized with MSSA, pseudomonas, burkholderia • • • Liver and kidney transplant 2014 DBD donor (‘donor after brain death’) • H/o post-transplant rejection x 1 treated with pulse steroids • • H/o BK viremia and CMV viremia • Diabetes 2/2 CF • Pancreatic exocrine failure Transplant Meds • Pulm Meds Dornase alpha daily • Levalbuterol prn • • Other Insulin glargine and aspart • Creon TID WM •

Social History: • No etoh, no smoking or other drugs • Lives with husband in Portland • No recent ill contacts • Works as a teacher • Social distancing Family History: • Noncontributory

WHAT IS YOUR DIFFERENTIAL DIAGNOSIS? WHAT’S MOST LIKELY? WHAT CAN’T YOU MISS?

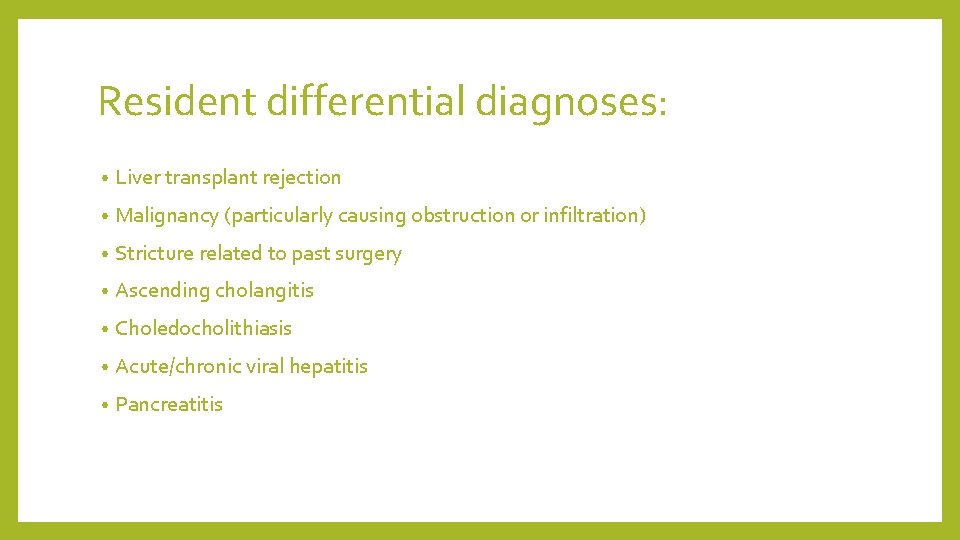
Resident differential diagnoses: • Liver transplant rejection • Malignancy (particularly causing obstruction or infiltration) • Stricture related to past surgery • Ascending cholangitis • Choledocholithiasis • Acute/chronic viral hepatitis • Pancreatitis

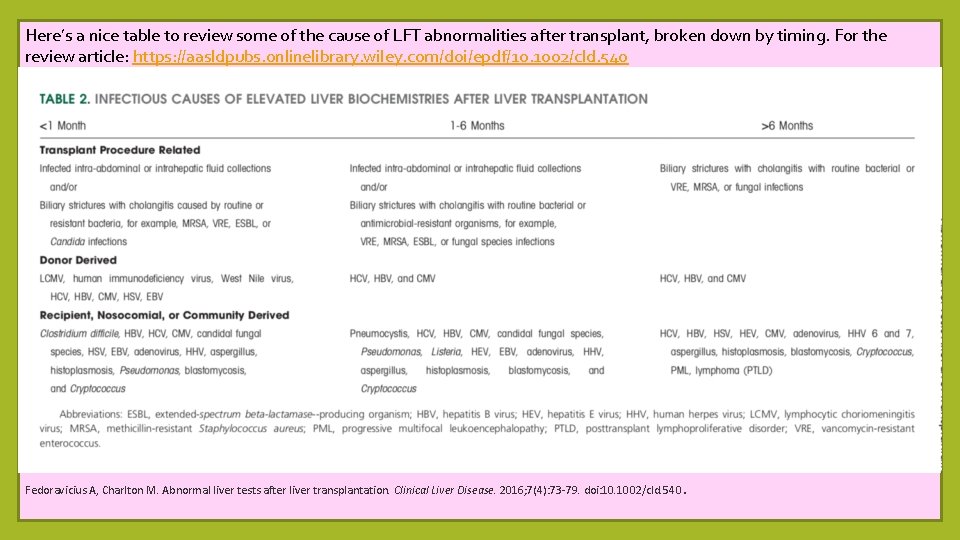
Here’s a nice table to review some of the cause of LFT abnormalities after transplant, broken down by timing. For the review article: https: //aasldpubs. onlinelibrary. wiley. com/doi/epdf/10. 1002/cld. 540 Fedoravicius A, Charlton M. Abnormal liver tests after liver transplantation. Clinical Liver Disease. 2016; 7(4): 73 -79. doi: 10. 1002/cld. 540. Fedoravicius A, Charlton M. Abnormal liver tests after liver transplantation. Clinical Liver Disease. 2016; 7(4): 73 -79. doi: 10. 1002/cld. 540.

BASED ON THE HPI AND HISTORY, WHAT DO YOU WANT TO LOOK FOR ON EXAM? WHY?

Exam: Vitals: BP 160/64, HR 80, T 98. 4, RR 16, O 2 100% Gen: Pleasant, no distress HEENT: + scleral icterus CV: RRR, no MRG Lungs: clear, unlabored breathing Abd: + tender in RUQ and RLQ, palpable spleen Skin: + jaundice Neuro: Alert, oriented x 4, no asterixis Lymph: no LAD

HOW HAS YOUR DIFFERENTIAL CHANGED WITH THE EXAM?

WHAT’S YOUR SUMMARY STATEMENT THUS FAR?

Resident’s summary statement: • 41 year old female with cystic fibrosis c/b cirrhosis further c/b HRS, now s/p liver and kidney transplant, on immunosuppression, presenting with acute onset jaundice and abdominal pain with exam notable for tenderness in RUQ and RLQ, overall concerning for biliary obstruction.

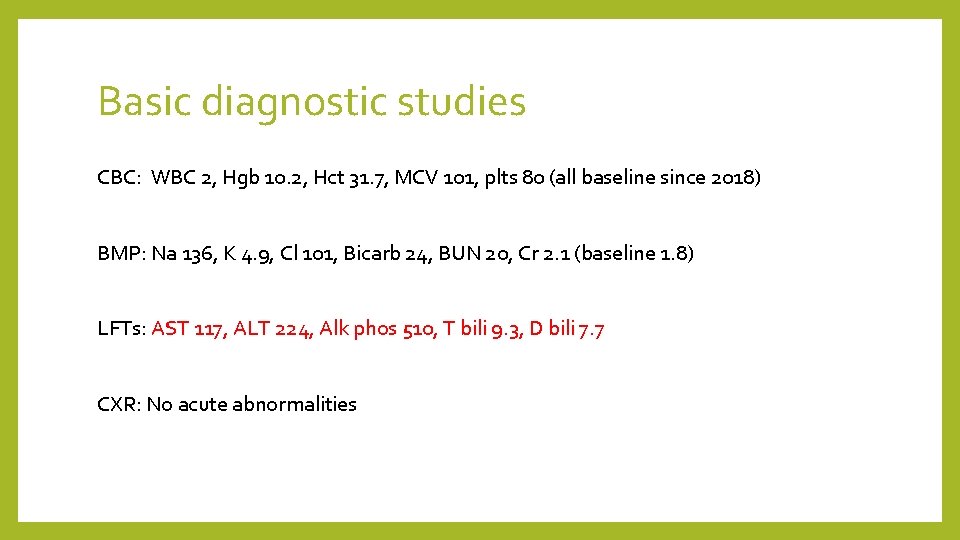
Basic diagnostic studies CBC: WBC 2, Hgb 10. 2, Hct 31. 7, MCV 101, plts 80 (all baseline since 2018) BMP: Na 136, K 4. 9, Cl 101, Bicarb 24, BUN 20, Cr 2. 1 (baseline 1. 8) LFTs: AST 117, ALT 224, Alk phos 510, T bili 9. 3, D bili 7. 7 CXR: No acute abnormalities

BASED ON YOUR DIFFERENTIAL, WHAT OTHER STUDIES DO YOU WANT? WHY?

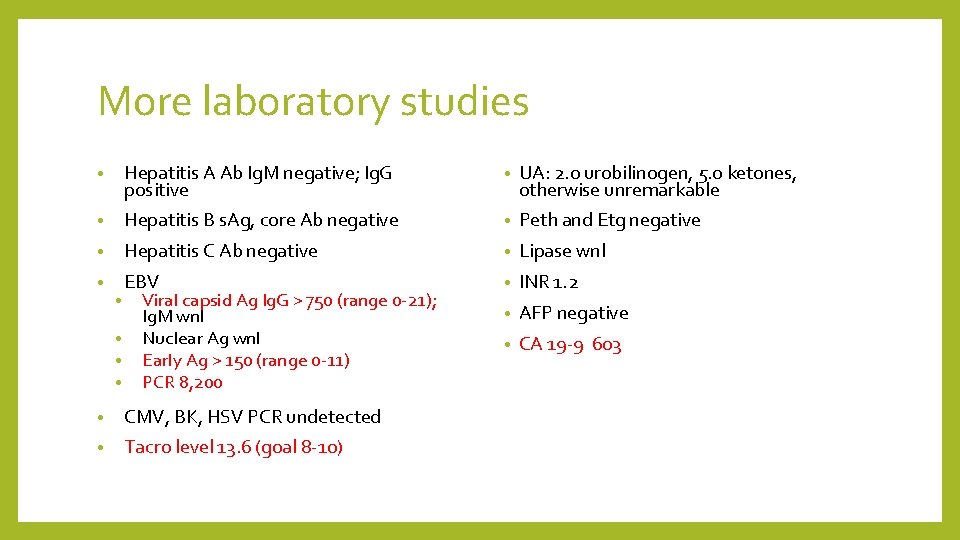
More laboratory studies • Hepatitis A Ab Ig. M negative; Ig. G positive • UA: 2. 0 urobilinogen, 5. 0 ketones, otherwise unremarkable • Hepatitis B s. Ag, core Ab negative • Peth and Etg negative • Hepatitis C Ab negative • Lipase wnl • EBV • INR 1. 2 • AFP negative • CA 19 -9 603 • • Viral capsid Ag Ig. G > 750 (range 0 -21); Ig. M wnl Nuclear Ag wnl Early Ag > 150 (range 0 -11) PCR 8, 200 • CMV, BK, HSV PCR undetected • Tacro level 13. 6 (goal 8 -10)

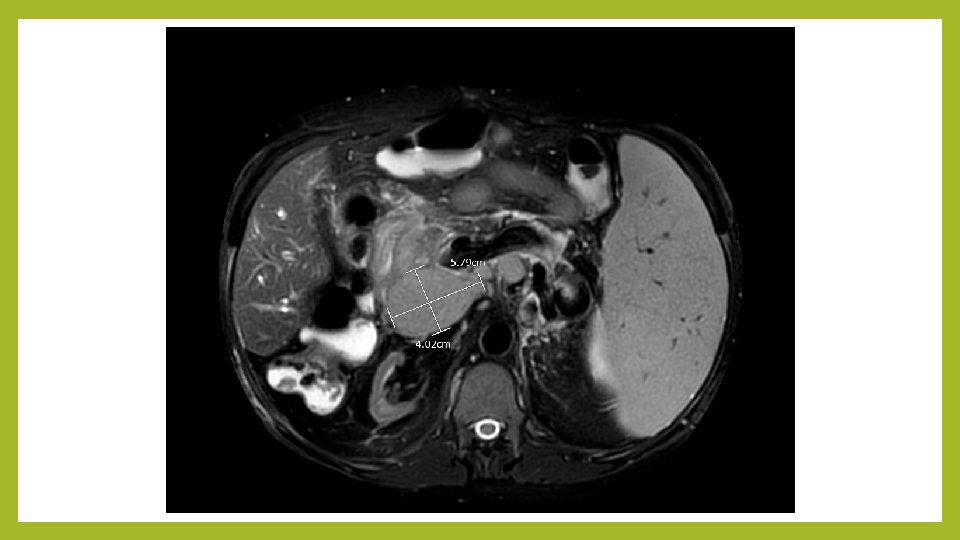
Imaging studies • Liver US: Diffuse severe intrahepatic biliary dilation and upper CBD dilation c/f obstruction • MRCP (no IV contrast 2/2 AKI): Mod- severe intrahepatic biliary dilatation. Innumerable cystic lesions along pancreatic duct. Complex solid and cystic lesion of the head of the pancreas measuring 2. 6 x 2. 1 cm. Markedly enlarged spleen, 16 cm. Atrophic kidneys without hydronephrosis. Extensive retroperitoneal and portal caval adenopathy. Numerous varices with numerous portosystemic collaterals.


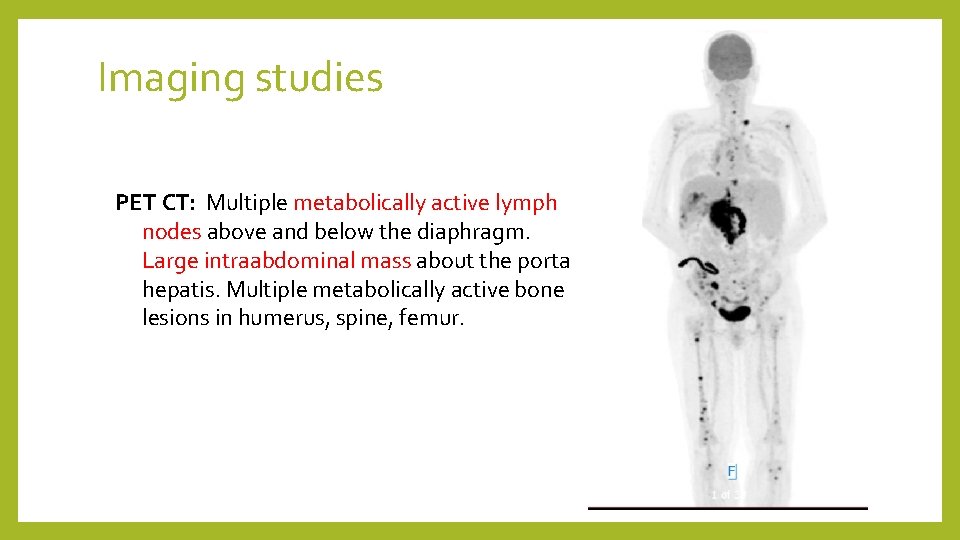
Imaging studies PET CT: Multiple metabolically active lymph nodes above and below the diaphragm. Large intraabdominal mass about the porta hepatis. Multiple metabolically active bone lesions in humerus, spine, femur.

WHAT IS YOUR LEADING DIAGNOSIS NOW? CAN WE STREAMLINE THE SUMMARY STATEMENT MORE WITH THIS DATA?

Resident’s modified summary statement: • 41 year old female with cystic fibrosis c/b cirrhosis further c/b HRS, s/p liver and kidney transplant on immunosuppression, presenting with acute onset jaundice and abdominal pain, with elevated LFTs (primarily cholestatic pattern), elevated tacrolimus level, highly positive EBV viremia and imaging findings of biliary dilatation, intraabdominal mass, and extensive adenopathy, overall most consistent with PTLD (post-transplant lymphoproliferative disorder), requiring biopsy.

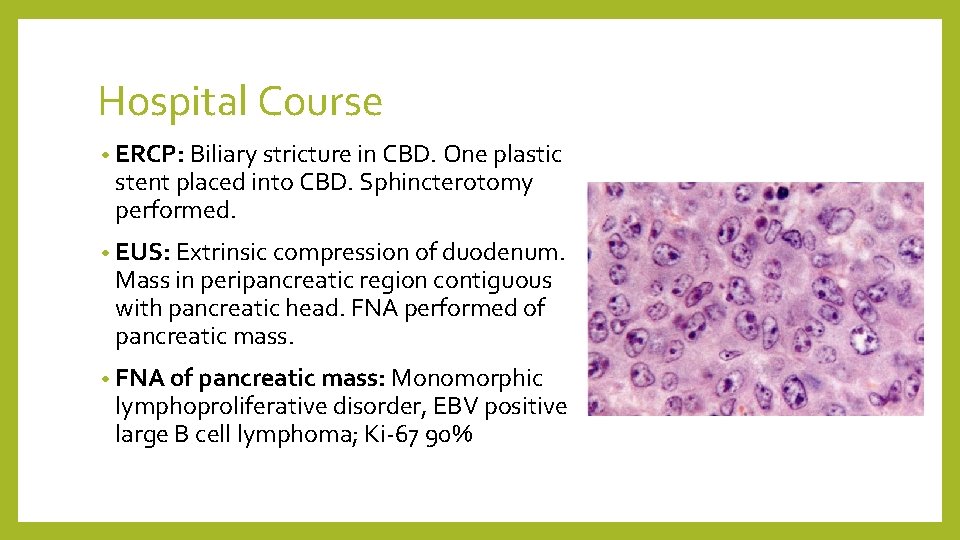
Hospital Course • ERCP: Biliary stricture in CBD. One plastic stent placed into CBD. Sphincterotomy performed. • EUS: Extrinsic compression of duodenum. Mass in peripancreatic region contiguous with pancreatic head. FNA performed of pancreatic mass. • FNA of pancreatic mass: Monomorphic lymphoproliferative disorder, EBV positive large B cell lymphoma; Ki-67 90%

Hospital Course • Bilirubin and LFTs downtrended after stent placed; jaundice, pain, and appetite improved • Decreased immunosuppression (significantly decreased tacrolimus dose) • Diagnosed with stage IV EBV + DLBCL • Transferred to malignant heme service to begin R-CHOP (rituximab, prednisone, vincristine, doxorubicin, cyclophosphamide)

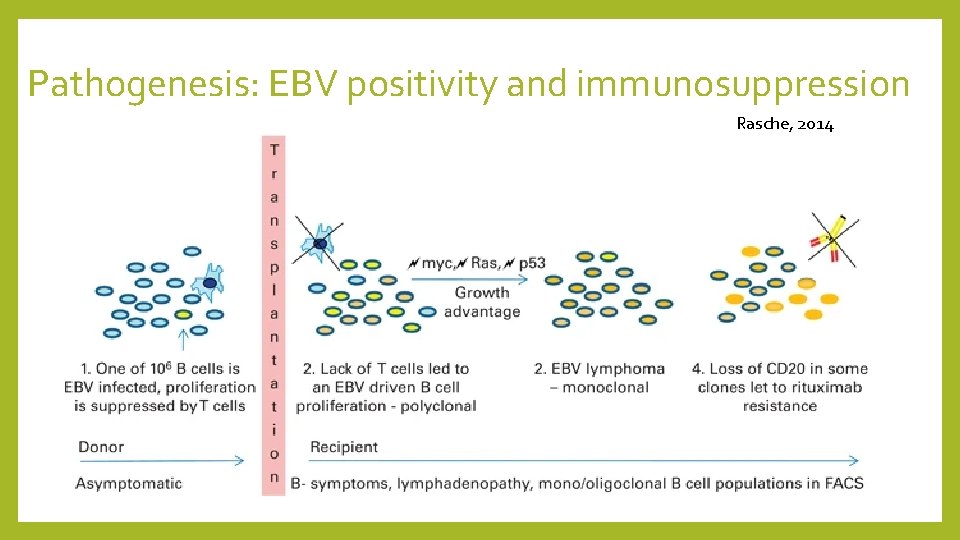
PTLD Overview For those interested, NEJM has a nice review article on PTLD: • Post-transplant lymphoproliferative disorder (PTLD) are lymphoid and/or plasmacytic proliferations that occur in the setting of solid organ or allogeneic hematopoietic cell transplantation as a result of immunosuppression. • Overall incidence of lymphoproliferative disease is approximately 1 percent in the transplant population • Majority are related to presence of Epstein-Barr virus (EBV) • Principal risk factors include 1. degree of T cell immunosuppression (recall her tacrolimus level was higher than goal) • 2. EBV serostatus of recipient (i. e. higher risk if recipient is EBV negative) • • Spectrum of PTLD types includes early lesions, polymorphic PTLD, and monomorphic PTLD

Spectrum of PTLD • Early lesions = plasmacytic hyperplasia and infectious mononucleosis-like PTLD (benign) • Polymorphic PTLD = demonstrate evidence of malignant transformation but do not meet all of the criteria for one of the B cell or T/NK cell lymphomas • Monomorphic PTLD = meet the criteria for one of the B cell or T/NK cell lymphomas (this is what our patient had)

Pathogenesis: EBV positivity and immunosuppression Rasche, 2014

PTLD Presentation and Diagnosis • • • Specific symptoms depend on areas of involvement • I. e. compression of surrounding structures (as in our patient, compression of duodenum and biliary system) Non-specific constitutional symptoms such as fever, weight loss, fatigue > 50 % of PTLD presents with extranodal masses • Our patient did have intraabdominal adenopathy and also had a discrete extranodal mass arising from the pancreas • Marked elevation in EBV viral load (hers was > 8, 000; that’s high) • Must have tissue!!

PTLD Management • Treatment depends on ‘type’ of PTLD (i. e. early, polymorphic, or monomorphic) • Since our patient has monomorphic PTLD, we’ll focus on that • Main initial treatment options are • 1. reduction in immunosuppression (optimal regimen not known; must take into consideration risk of graft rejection) • 2. rituximab (for CD 20+ disease) • 3. chemo • 4. radiation • 5. combo of above • All patients (with appropriate performance status) with CD 20+ monomorphic PTLD are offered rituximab plus combination chemotherapy (eg, CHOP)

MKSAP QUESTION

Which of the following is the most likely cause of her clinical findings? A) Cytomegalovirus infection B) Invasive candidal infection C) Posttransplant lymphoproliferative disorder D) Reactivation tuberculosis

Which of the following is the most likely cause of her clinical findings? A) Cytomegalovirus infection B) Invasive candidal infection C) Posttransplant lymphoproliferative disorder D) Reactivation tuberculosis

EXPLANATION: • This patient most likely has posttransplant lymphoproliferative disease (PTLD). She is at increased risk for PTLD because she was seronegative for Epstein-Barr virus (EBV) and the organ donor was seropositive. PTLD is related to B-cell proliferation induced by infection with EBV in the setting of chronic immunosuppression and resulting decreased T-cell immune surveillance. She presents within a typical timeframe (first few months to 1 year after transplantation) with lymphadenopathy found on CT scan and systemic symptoms consistent with PTLD. In addition to PTLD, EBV after transplantation cause a mononucleosis syndrome, leukopenia and thrombocytopenia, and hepatitis or pneumonitis. Quantitative serum polymerase chain reaction for EBV can suggest a diagnosis of PTLD, which is confirmed by biopsy and histopathologic evaluation of the swollen lymph nodes or extranodal mass. Management consists of reduction in immunosuppression, if possible, and may require chemotherapy, which often includes rituximab. • Cytomegalovirus infection is common after transplantation, especially in seronegative recipients with seropositive donors, and can manifest as a nonspecific febrile syndrome. However, cytomegalovirus would be unlikely to cause a single, significantly enlarged lymph node; this presentation makes PTLD more likely. • Although this patient has whitish plaques in her oropharynx consistent with oral thrush, and disseminated fungal infections may cause systemic symptoms such as this patient is experiencing, an invasive Candida infection would not cause her isolated lymphadenopathy and is not supported by her negative fungal blood cultures. • Tuberculosis reactivation increases in incidence after solid organ transplantation and is more likely to have extrapulmonary findings. It could present with lymphadenopathy and fever but clear lungs, as in this patient. However, she had a negative result on a test for latent tuberculosis before transplantation, which makes reactivation of tuberculosis unlikely.


• Please leave a comment/take away learning point on the blog!!!!

References • Abbas F 1, El Kossi M 2, Shaheen IS 2, Sharma Post-transplantation lymphoproliferative disorders: Current concepts and future therapeutic approaches. World J Transplant. 2020 Feb 28; 10(2): 29 -46. doi: 10. 5500/wjt. v 10. i 2. 29. • A 2, Halawa A 2. Nagle SJ 1, Reshef R 2, Tsai DE 3. Posttransplant Lymphoproliferative Disorder in Solid Organ and Hematopoietic Stem Cell Transplantation. Clin Chest Med. 2017 Dec; 38(4): 771783. doi: 10. 1016/j. ccm. 2017. 08. 001. Epub 2017 Sep 21. • Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, Marcus R, Parameshwar J, Ramsay A, Newstead C, Haemato-oncology Task Force of the British Committee for Standards in Haematology and British Transplantation Society. Br J Haematol. 2010; 149(5): 693. Epub 2010 Apr 16 • Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS Guidelines. • Rasche, L. , Kapp, M. , Einsele, H. et al. EBV-induced post transplant lymphoproliferative disorders: a persisting challenge in allogeneic hematopoetic SCT. Bone Marrow Transplant 49, 163– 167 (2014). https: //doi. org/10. 1038/bmt. 2013. 96.
 Obstructive jaundice vs hemolytic jaundice
Obstructive jaundice vs hemolytic jaundice Porters generic competitive strategies
Porters generic competitive strategies Timex cost leadership strategy
Timex cost leadership strategy Actor focus vs object focus
Actor focus vs object focus Focus on form vs focus on forms
Focus on form vs focus on forms Sleep diagnostics butte mt
Sleep diagnostics butte mt Mitel system administration and diagnostics download
Mitel system administration and diagnostics download Standard diagnostics inc
Standard diagnostics inc Molecular diagnostics
Molecular diagnostics Dbghost.exe
Dbghost.exe Birads scoring
Birads scoring Duct diagnostics
Duct diagnostics Duct diagnostics
Duct diagnostics Bio synex
Bio synex Hiv diagnostics conference
Hiv diagnostics conference Kcf smart diagnostics
Kcf smart diagnostics Da toolset
Da toolset Realtime diagnostics
Realtime diagnostics Spss casewise diagnostics
Spss casewise diagnostics Felice shieh
Felice shieh Cole diagnostics jobs
Cole diagnostics jobs Customer journey diagnostics
Customer journey diagnostics Duct diagnostics
Duct diagnostics Aff definitioner
Aff definitioner Connective tissue
Connective tissue Laser beam diagnostic
Laser beam diagnostic Teleradiology
Teleradiology Sensiringe
Sensiringe Emc diagnostics
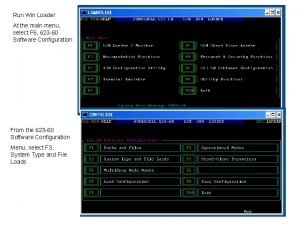
Emc diagnostics Winloader
Winloader Maximum number of diagnostics exceeded
Maximum number of diagnostics exceeded Computational diagnostics
Computational diagnostics Diagnostics infirmier
Diagnostics infirmier Duct diagnostics
Duct diagnostics Gm strategy based diagnostics chart
Gm strategy based diagnostics chart Misyonerong dumating noong 1587
Misyonerong dumating noong 1587