NONSTEADY STATE DIFFUSION Nonsteady state The concentration profile
- Slides: 5

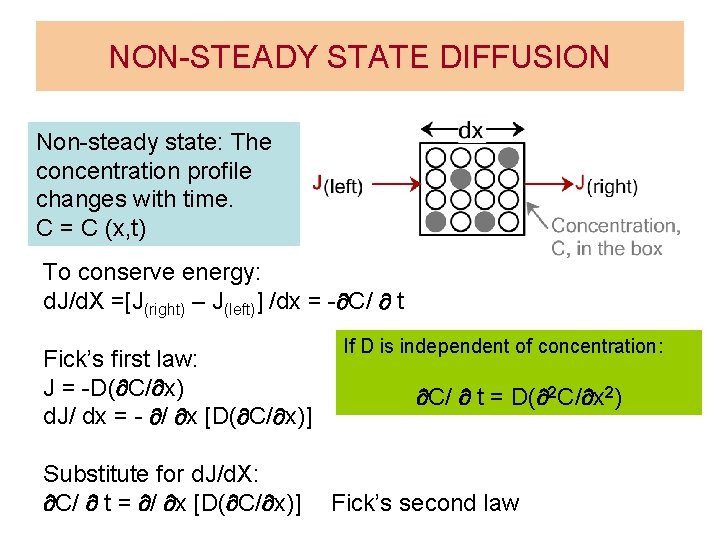
NON-STEADY STATE DIFFUSION Non-steady state: The concentration profile changes with time. C = C (x, t) To conserve energy: d. J/d. X =[J(right) – J(left)] /dx = - C/ t Fick’s first law: J = -D( C/ x) d. J/ dx = - / x [D( C/ x)] Substitute for d. J/d. X: C/ t = / x [D( C/ x)] If D is independent of concentration: C/ t = D( 2 C/ x 2) Fick’s second law

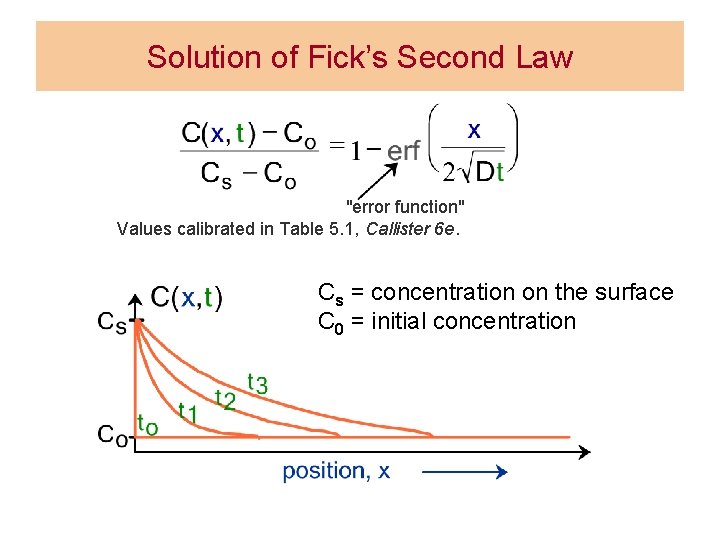
Solution of Fick’s Second Law "error function" Values calibrated in Table 5. 1, Callister 6 e. Cs = concentration on the surface C 0 = initial concentration

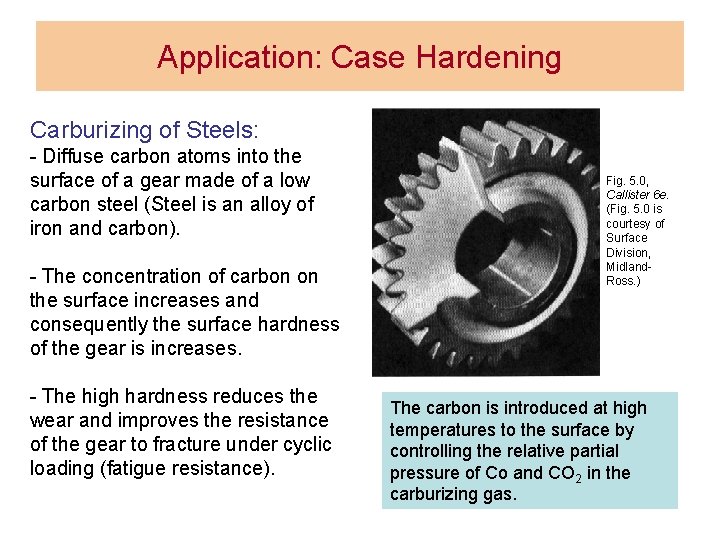
Application: Case Hardening Carburizing of Steels: - Diffuse carbon atoms into the surface of a gear made of a low carbon steel (Steel is an alloy of iron and carbon). - The concentration of carbon on the surface increases and consequently the surface hardness of the gear is increases. - The high hardness reduces the wear and improves the resistance of the gear to fracture under cyclic loading (fatigue resistance). Fig. 5. 0, Callister 6 e. (Fig. 5. 0 is courtesy of Surface Division, Midland. Ross. ) The carbon is introduced at high temperatures to the surface by controlling the relative partial pressure of Co and CO 2 in the carburizing gas.

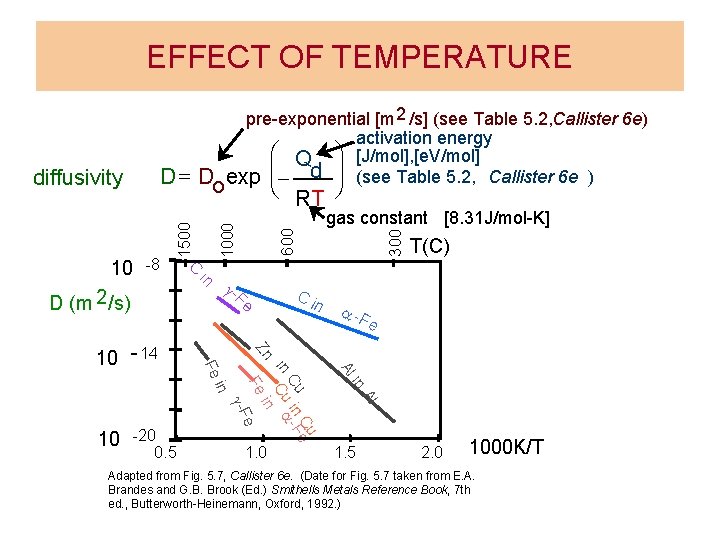
EFFECT OF TEMPERATURE Fe n a -F e in Al Al Cue Cu in -F in Cu a in Fe e g -F -20 0. 5 T(C) Zn in 10 Ci g- Fe 10 - 14 300 600 in D (m 2 /s) gas constant [8. 31 J/mol-K] 1000 -8 è RT ø C 10 1500 D = Do diffusivity pre-exponential [m 2 /s] (see Table 5. 2, Callister 6 e) activation energy æ Q ö [J/mol], [e. V/mol] exp ç- d ÷ (see Table 5. 2, Callister 6 e ) 1. 0 1. 5 2. 0 1000 K/T Adapted from Fig. 5. 7, Callister 6 e. (Date for Fig. 5. 7 taken from E. A. Brandes and G. B. Brook (Ed. ) Smithells Metals Reference Book, 7 th ed. , Butterworth-Heinemann, Oxford, 1992. )

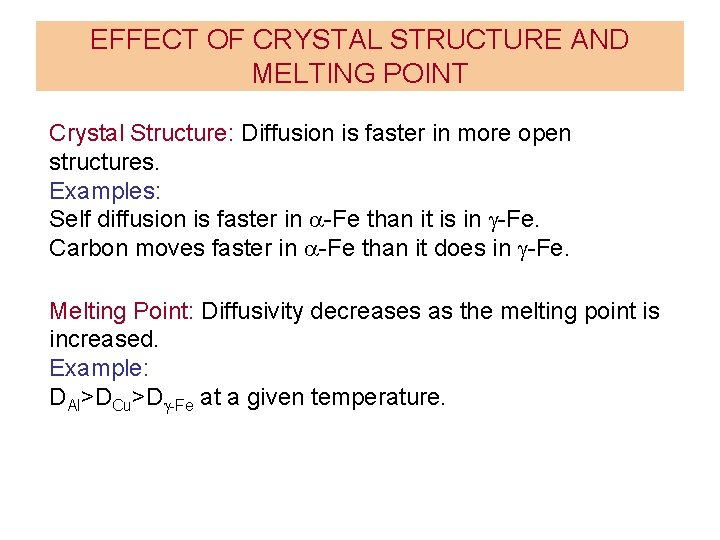
EFFECT OF CRYSTAL STRUCTURE AND MELTING POINT Crystal Structure: Diffusion is faster in more open structures. Examples: Self diffusion is faster in a-Fe than it is in g-Fe. Carbon moves faster in a-Fe than it does in g-Fe. Melting Point: Diffusivity decreases as the melting point is increased. Example: DAl>DCu>Dg-Fe at a given temperature.