NonInsulin Diabetes Medications LAURA SHANEMCWHORTER PHARMD BCPS BCADM

Non-Insulin Diabetes Medications LAURA SHANE-MCWHORTER, PHARMD, BCPS, BC-ADM, CDE, FASCP, FAADE

Learning Objectives Know the mechanism of action, side effects, and drug interactions of non-insulin agents for diabetes Compare the efficacy of different medications to treat T 2 DM State which agents are oral and which agents are injectable Formulate a therapeutic regimen for T 2 DM based on aligning the mechanism of action of diabetes medications with the pathophysiologic changes Design a plan to add insulin to oral agents in T 2 DM
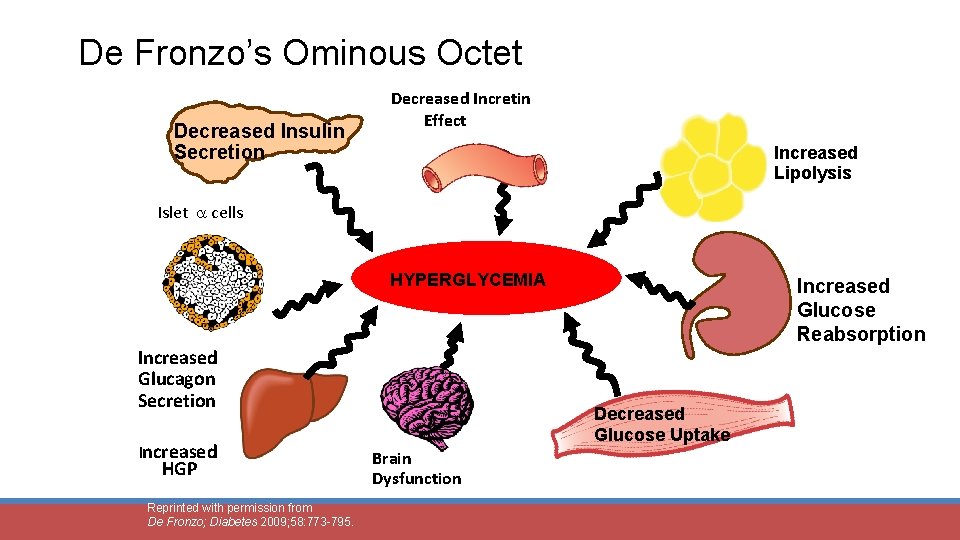
De Fronzo’s Ominous Octet Decreased Insulin Secretion Decreased Incretin Effect Increased Lipolysis Islet cells HYPERGLYCEMIA Increased Glucagon Secretion Increased HGP Reprinted with permission from De Fronzo; Diabetes 2009; 58: 773 -795. Increased Glucose Reabsorption Decreased Glucose Uptake Brain Dysfunction
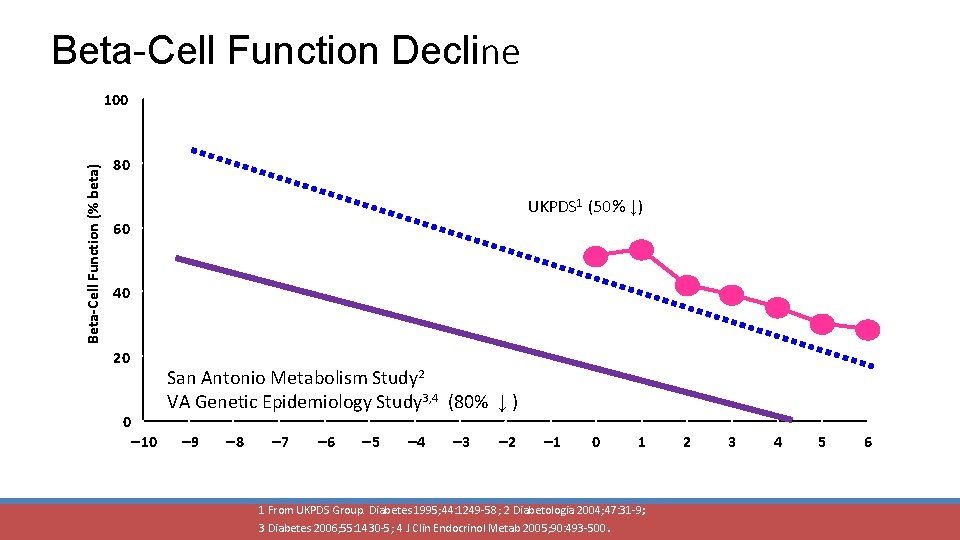
Beta-Cell Function Decline Beta-Cell Function (% beta) 100 80 UKPDS 1 (50% ↓) 60 40 20 0 San Antonio Metabolism Study 2 VA Genetic Epidemiology Study 3, 4 (80% ↓ ) 10 9 8 7 6 5 4 3 Years 2 1 0 1 1 From UKPDS Group. Diabetes 1995; 44: 1249 -58; 2 Diabetologia 2004; 47: 31 -9; 3 Diabetes 2006; 55: 1430 -5; 4 J Clin Endocrinol Metab 2005; 90: 493 -500. 2 3 4 5 6
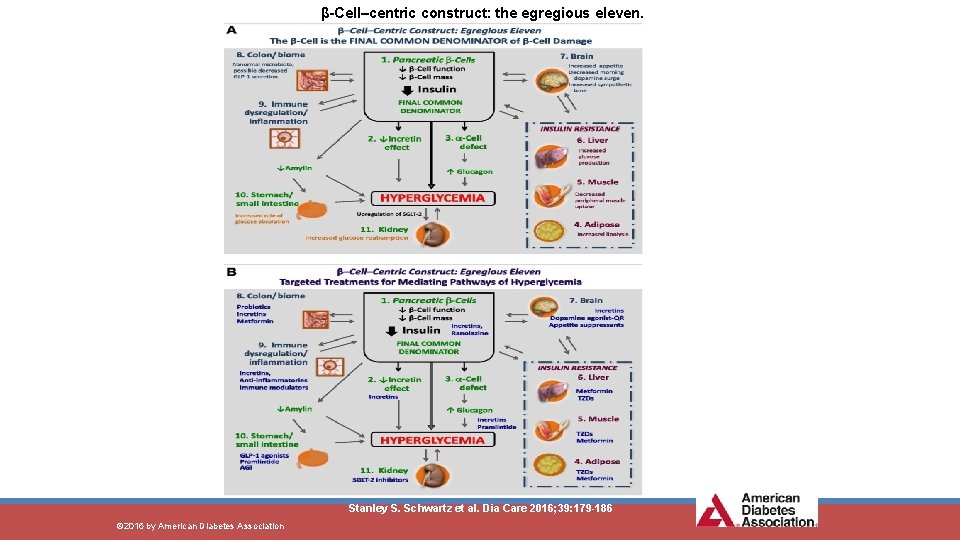
β-Cell–centric construct: the egregious eleven. Stanley S. Schwartz et al. Dia Care 2016; 39: 179 -186 © 2016 by American Diabetes Association
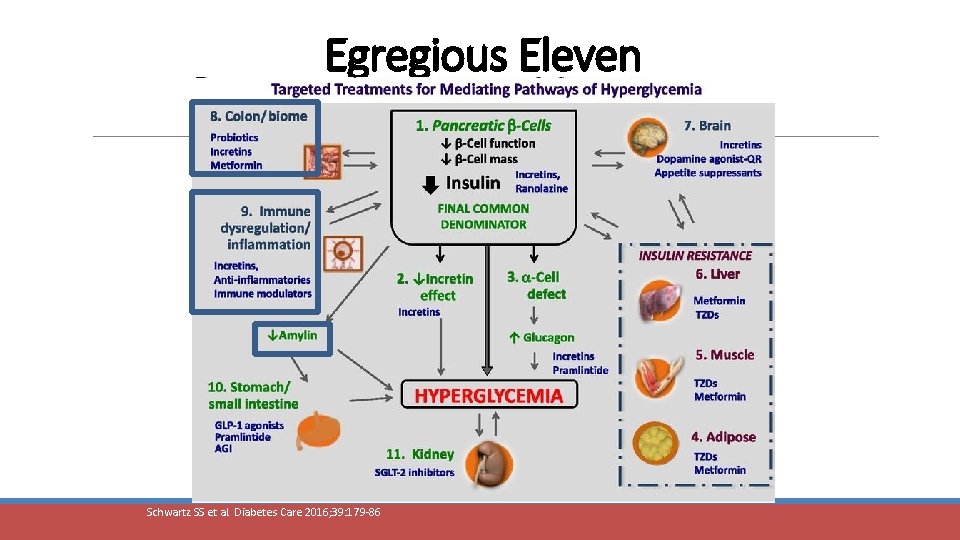
Egregious Eleven Schwartz SS et al. Diabetes Care 2016; 39: 179 -86

Non-Insulin Medications for T 2 DM Metformin Sulfonylureas Glinides (meglitinides) Thiazolidinediones (TZDs or glitazones) α-Glucosidase inhibitors Colesevelam Bromocriptine SGLT-2 Inhibitors Pramlintide (Injectable used in Type 1 and Type 2 DM) GLP-1 analogs (Injectable) DPP-IV inhibitors
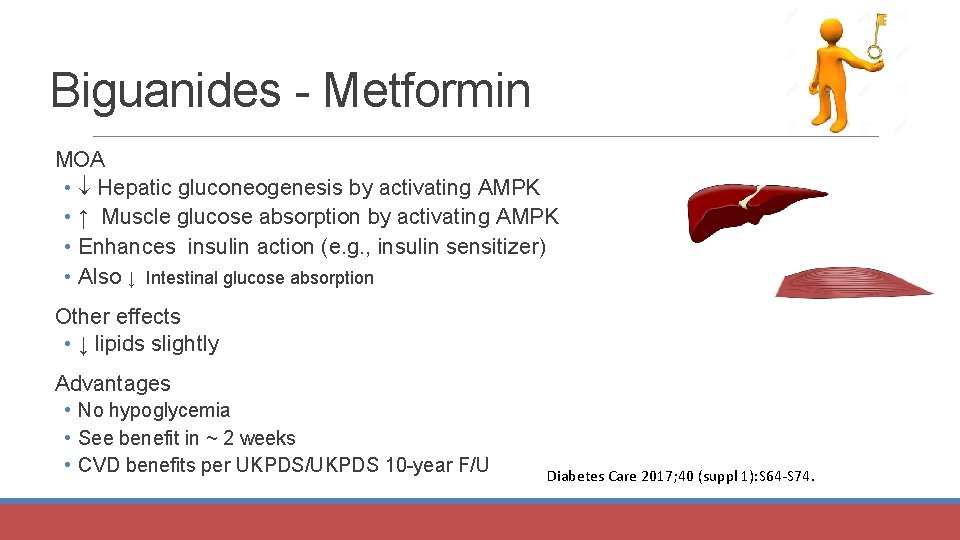
Biguanides - Metformin MOA • Hepatic gluconeogenesis by activating AMPK • ↑ Muscle glucose absorption by activating AMPK • Enhances insulin action (e. g. , insulin sensitizer) • Also ↓ Intestinal glucose absorption Other effects • ↓ lipids slightly Advantages • No hypoglycemia • See benefit in ~ 2 weeks • CVD benefits per UKPDS/UKPDS 10 -year F/U Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
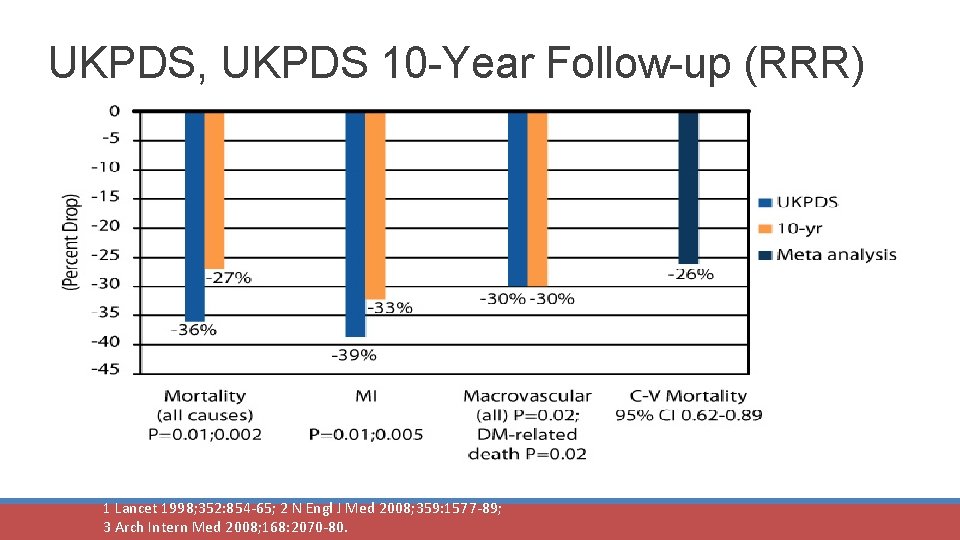
UKPDS, UKPDS 10 -Year Follow-up (RRR) Mortality (all-cause) P=0. 01; 0. 002 MI P=0. 01; 0. 005 Macrovascular DM-related (all; P=0. 02) death P=0. 02 1 Lancet 1998; 352: 854 -65; 2 N Engl J Med 2008; 359: 1577 -89; 3 Arch Intern Med 2008; 168: 2070 -80. C-V Mortality 95% CI 0. 62 -0. 89

Biguanides - Metformin Side Effects/Limitations • GI adverse effects (titrate slowly) • In renal dysfunction must dose § § § SCr, GFR 45 to 60 m. L/min – use at full dose to half at GFR 45 m. L/min Discontinue at GFR 30 m. L/min • Lactic acidosis risk (Binge drinking, severe renal dysfunction, radiocontrast media) • B 12 deficiency with long-term use • Caution in females of childbearing age Dose • Start at 500 mg qd and titrate up to 2000 mg/day Effects • FPG 50 -60 mg/d. L • A 1 C – 1%-2% http: //www. fda. gov/Safety/Med. Watch/Safety. Information/Safety. Alertsfor. Human. Medical. Pr oducts/ucm 494829. htm? source=govdelivery&utm_medium=email&utm_source=govdeliver y

QM QM is a 52 y/o male with T 2 DM. He has a Cr of 1. 6 mg/d. L and his GFR is 55. • Would you use metformin? What dose? What if QM had a Cr of 1. 6 mg/d. L and his GFR is 40. • Would you use metformin? What dose?
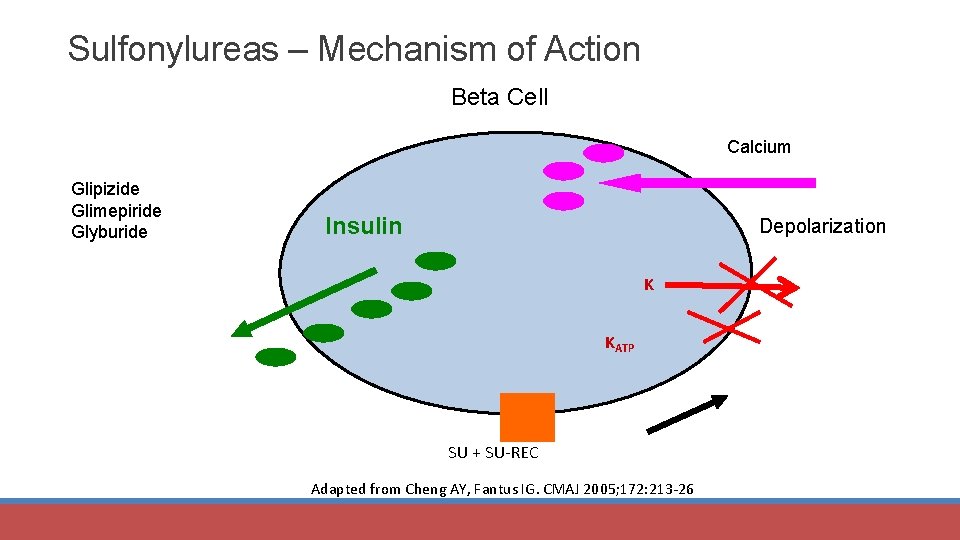
Sulfonylureas – Mechanism of Action Beta Cell Calcium Glipizide Glimepiride Glyburide Insulin Depolarization K KATP SU + SU-REC Adapted from Cheng AY, Fantus IG. CMAJ 2005; 172: 213 -26.

Sulfonylureas MOA: • Stimulate insulin secretion § Close KATP channels on beta-cell plasma membranes (blocks K efflux) § Beta-cell depolarization § Calcium channels open; influx of calcium § Efflux of insulin Uses: monotherapy or combination Most used: glipizide, glimepiride, glyburide Advantages • See benefit in 2 weeks • Target impaired insulin secretion • Inexpensive Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
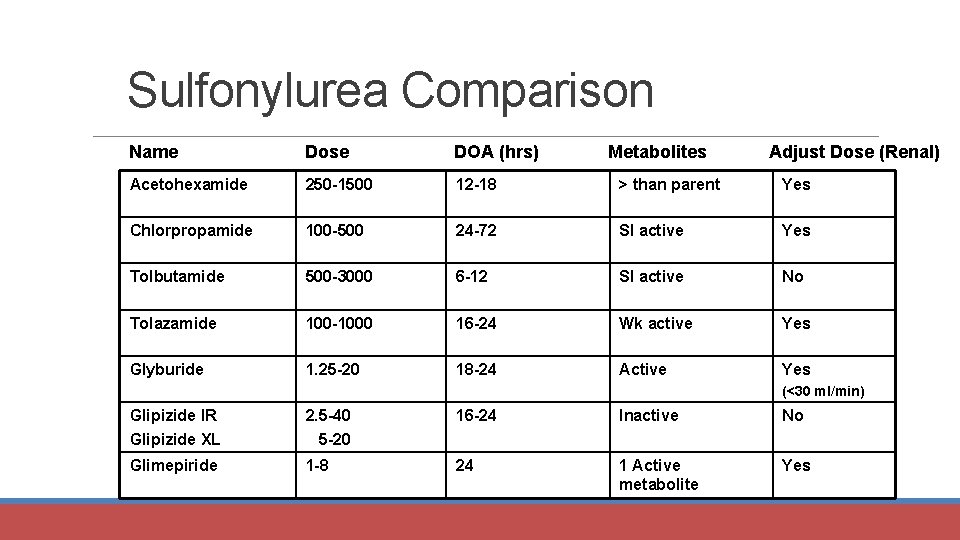
Sulfonylurea Comparison Name Dose DOA (hrs) Metabolites Adjust Dose (Renal) Acetohexamide 250 -1500 12 -18 > than parent Yes Chlorpropamide 100 -500 24 -72 Sl active Yes Tolbutamide 500 -3000 6 -12 Sl active No Tolazamide 100 -1000 16 -24 Wk active Yes Glyburide 1. 25 -20 18 -24 Active Yes (<30 ml/min) Glipizide IR Glipizide XL 2. 5 -40 5 -20 16 -24 Inactive No Glimepiride 1 -8 24 1 Active metabolite Yes

Sulfonylureas Side Effects/Limitations • Weight gain • Hypoglycemia (don’t delay/skip meals) § Elderly § Renal function § Dosing issues • Start at low doses and titrate up carefully • Max benefit at half of max doses • 5%-15% yearly secondary failure Effects • Mixed: in FPG, PPG • glucose 50 -60 mg/d. L • A 1 C – 1%-2% Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
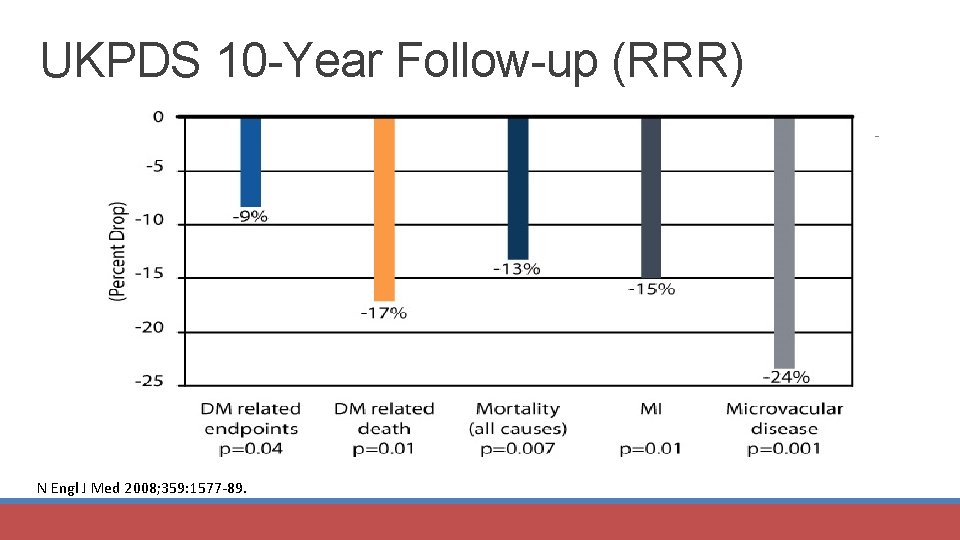
UKPDS 10 -Year Follow-up (RRR) N Engl J Med 2008; 359: 1577 -89.

Glinides Repaglinide (Prandin®), Nateglinide (Starlix®) MOA: Same as sulfonylurea (release insulin) Uses: Monotherapy or combination Advantages • Less hypoglycemia than sulfonylureas (short half-life, DOA) • PPG • May use in renal function Side Effects/Limitations • Weight • Three times/day dosing Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
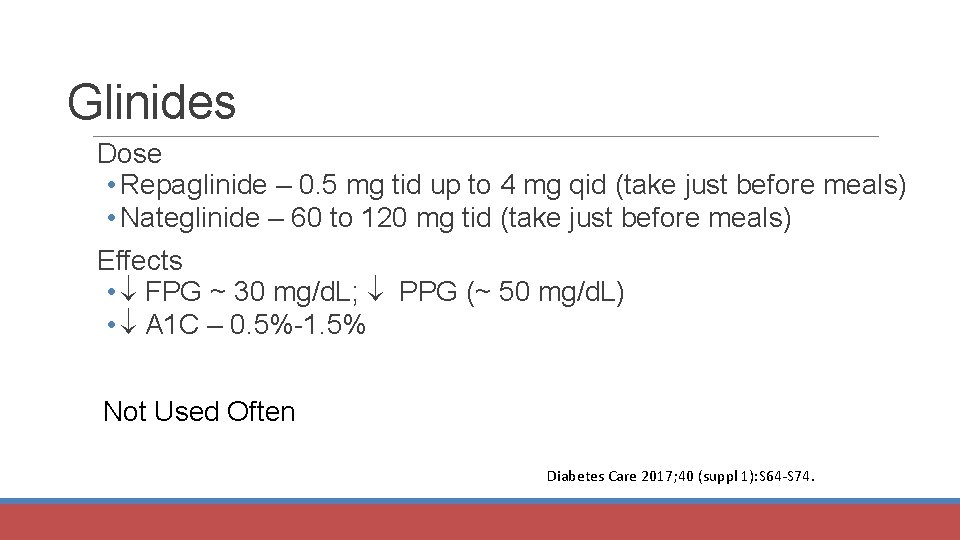
Glinides Dose • Repaglinide – 0. 5 mg tid up to 4 mg qid (take just before meals) • Nateglinide – 60 to 120 mg tid (take just before meals) Effects • FPG ~ 30 mg/d. L; PPG (~ 50 mg/d. L) • A 1 C – 0. 5%-1. 5% Not Used Often Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

Thiazolidinediones Pioglitazone (Actos®), Rosiglitazone (Avandia®) MOA: • Activate nuclear transcription factor - PPAR- § Potent increase in insulin sensitivity in muscle, fat, liver Monotherapy or combination Advantages • No hypoglycemia • Improves lipids (↑ HDL, ↓ TGs - pioglitazone) • ↑ Insulin sensitivity • Durability Diabetes Care 2017; 40 (suppl 1): S 64 -S 74; Diabetes 2009; 58: 773 -95.
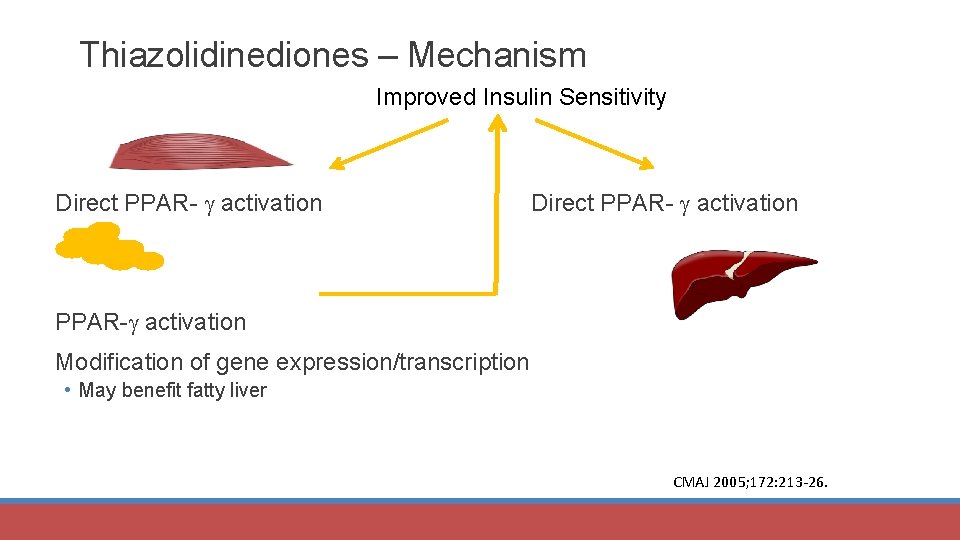
Thiazolidinediones – Mechanism Improved Insulin Sensitivity Direct PPAR- activation PPAR- activation Modification of gene expression/transcription • May benefit fatty liver CMAJ 2005; 172: 213 -26.

Thiazolidinediones Side Effects/Limitations • Fluid retention/weight gain • HF/cardiac events • ↑ Fracture risk • ↑ MI (rosiglitazone) • ↑ Bladder cancer (pioglitazone) • Effect takes several weeks. Dose • Pioglitazone: 15 -45 mg/day • Rosiglitazone: 4 -8 mg/day Effects • FPG 20 -40 mg/d. L • A 1 C – 0. 5%-1. 4% Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
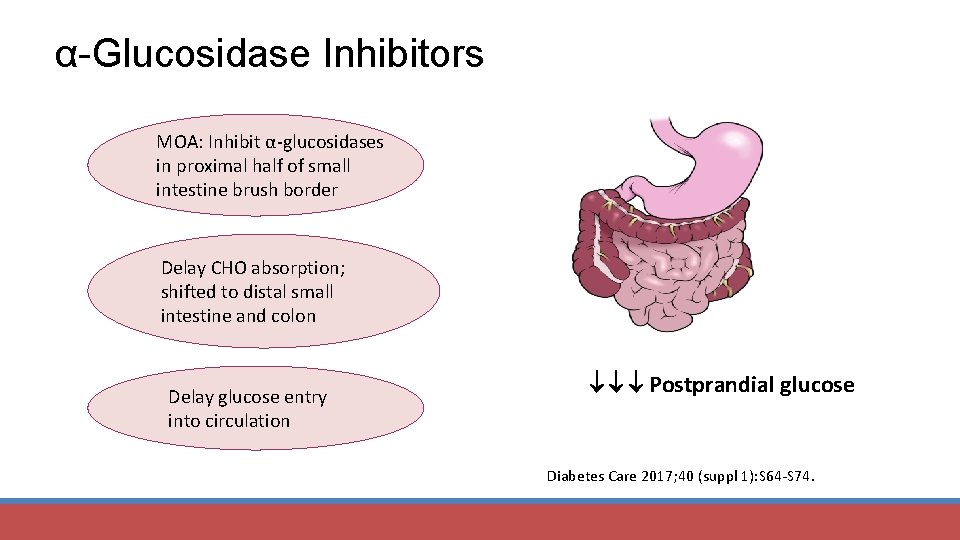
α-Glucosidase Inhibitors MOA: Inhibit α-glucosidases in proximal half of small intestine brush border Delay CHO absorption; shifted to distal small intestine and colon Delay glucose entry into circulation Postprandial glucose Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
α-Glucosidase Inhibitors Examples: Acarbose (Precose®) and Miglitol (Glyset®) MOA: Inhibit intestinal brush border enzymes that break down saccharides • e. g. , CHO absorption Monotherapy or combination Advantages • Weight neutral • Blunts PPG and glucose AUC • Has shown DM onset/improved CV outcomes Side Effects/Limitations • GI adverse effects • Slow titration Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
α-Glucosidase Inhibitors Dose • 25 mg with food (weeks 1 -2) • 25 mg bid with food (weeks 3 -4) • 25 mg tid with food (weeks 5 -12) • 50 mg tid if 60 kg or less; 100 mg tid if > 60 kg Effects • FPG 20 mg/d. L; PPG ~ 50 mg/d. L • A 1 C – 0. 5%-0. 8% Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

Bile Acid Sequestrant Colesevelam? (Welchol®) May add to oral agents or insulin Mechanism? • Blocks glucose absorption? • Binds bile acids • Activation of farnesoid x receptor in liver • Inhibition of cholecystokinin release § ↑ incretin levels? Diabetes Care 2017; 40 (suppl 1): S 64 -S 74; Curr Med Res Opin 2007; 23: 1673 -84; Drugs 2007; 67: 1383 -92.
Bile Acid Sequestrant Advantages • Not systemically absorbed • No hypoglycemia • ↓ LDL (12%-16%) Disadvantages • Constipation, nausea, dyspepsia • ↑ Triglycerides (~ 16 mg/d. L) § Contraindicated if TG > 500 mg/d. L • Must take other meds 4 hours BEFORE colesevelam to prevent binding Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
Bile Acid Sequestrant Dose • 3750 mg/day • Take 3 tablets of 625 mg each twice daily with lunch/supper • OR… 6 tabs with supper daily Effects • FPG ~ 14 mg/d. L • A 1 C – 0. 5% Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
Dopamine Agonist - Bromocriptine Mechanism 1 • Activates DA receptors § Thus results in other effects (FYI: ↓ sympathetic nervous system stimulation, hepatic glucose production, lipolysis/FFA, lipogenesis/TGs) • Alters hypothalamic regulation of metabolism • May ↑ insulin sensitivity • “Resets” biological clock to reverse metabolic changes associated with insulin resistance/obesity (no ↑ insulin) Advantages • No hypoglycemia; weight loss (or weight neutral) • ↓ Postprandial FFAs, TGs 1 Diabetes Care 2011; 34: 789 -94

Dopamine Agonist - Bromocriptine Side Effects/Limitations • Takes several weeks to see effects (12 -16 weeks) • Hallucinations, nausea, hypotension, fatigue • Contraindicated in women who are nursing • Must dose in morning § If dose is missed, wait until next day Drug interactions • Dopamine antagonists • Antihypertensives • Highly protein-bound drugs • CYP 3 A 4 substrate Diabetes Care 2011; 34: 789 -94; JFP (online) 2011; 60: E 1 -E 5; Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

Dopamine Agonist - Bromocriptine Dose • 0. 8 mg qd • Titrate by 0. 8 mg q week to max tolerated dose of 1. 6 to 4. 8 mg/day Effects • FPG ~ 8 -10 mg/d. L • A 1 C – 0. 5% Diabetes Care 2011; 34: 789 -94; JFP (online) 2011; 60: E 1 -E 5.
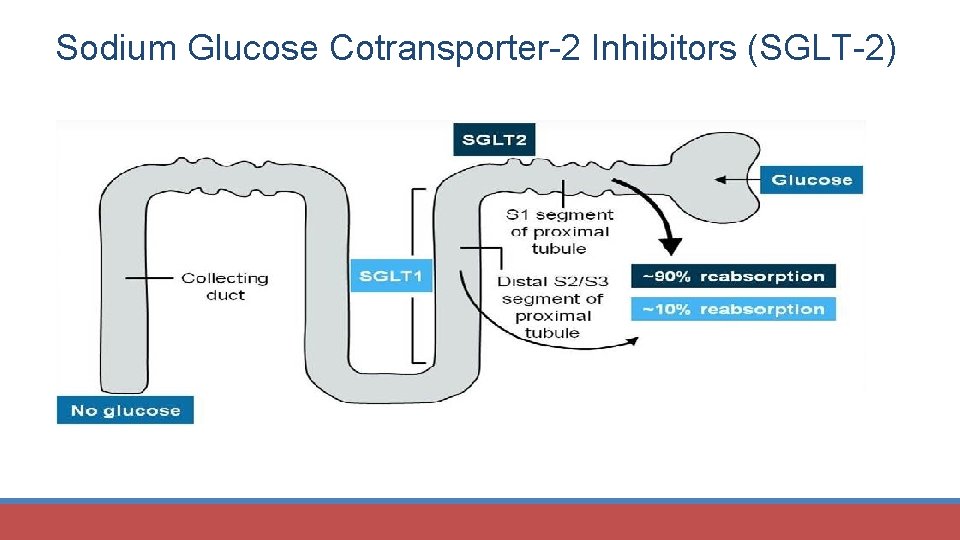
Sodium Glucose Cotransporter-2 Inhibitors (SGLT-2)

SGLT-2 Inhibitors SGLT 1 and SGLT 2 cause glucose reabsorption • SGLT 1 in gut; 10% of glucose reabsorption • SGLT 2 in proximal tubule; 90% of glucose reabsorption Canagliflozin (Invokana®) Dapagliflozin (Farxiga®) Empagliflozin (Jardiance®) Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

SGLT-2 Inhibitors MOA • Inhibition of glucose reabsorption in proximal nephron • �urinary glucose excretion • Subsequent �in PG Side effects/Limitations ($$$$) • • • Yeast infections UTIs Hypotension/volume depletion/dizziness (don’t use if hypovolemic) Hypoglycemia (with secretagogues) �potassium; amputations (canagliflozin) Bladder cancer (dapagliflozin) �LDL slightly (all) �creatinine slightly (all) Euglycemic ketoacidosis (all) Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

SGLT-2 Inhibitors Drug interactions • Inducers § �SDCs • ACE inhibitors? § �Potassium (canagliflozin) • Diuretics? § �BP • NSAIDs may �dapagliflozin SDCs Benefits • �weight (1 -3%) • �BP • Empagliflozin – 2016 FDA approved indication: prevention of death due to CVD in adults with T 2 DM and existing CVD 1 N Engl J Med 2015; 373: 2117 -28
SGLT-2 Inhibitors Cautions • Evaluate volume status; don’t use if hypovolemic • Caution if on diuretics • Contraindicated: severe renal dysfunction, dialysis § Canagliflozin: dose is 100 mg qam ac; may �to 300 mg qam ac • Don’t use if GFR < 45 m. L/min § Empagliflozin: dose is 10 mg qam ac; may �to 25 mg qam ac • Don’t use if GFR < 45 m. L/min § Dapagliflozin: dose is 5 mg qam ac; may �to 10 mg qam ac • Don’t use if GFR < 60 m. L/min Impact on glucose lowering/A 1 C • �FPG ~ 25 mg/d. L • �A 1 C ~ 1% Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
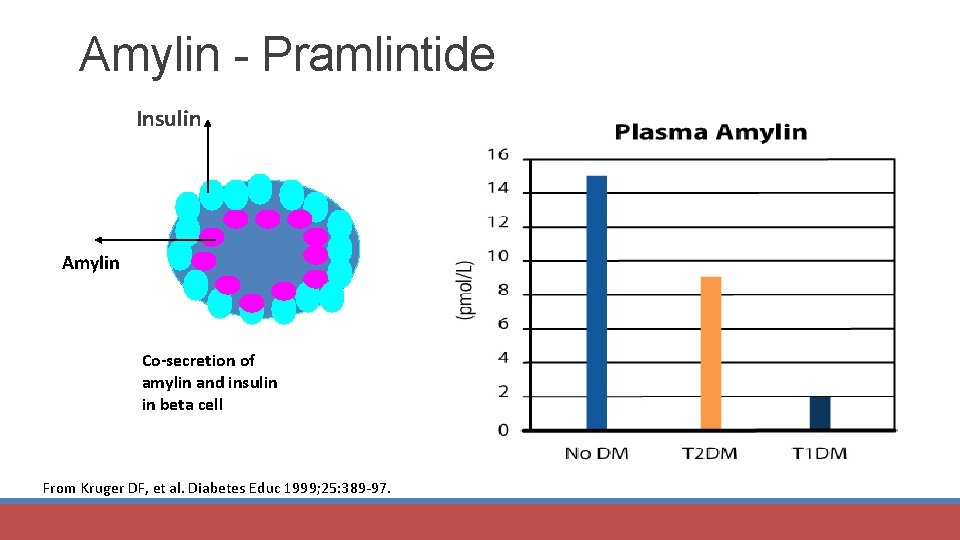
Amylin - Pramlintide Insulin Amylin Co-secretion of amylin and insulin in beta cell From Kruger DF, et al. Diabetes Educ 1999; 25: 389 -97.
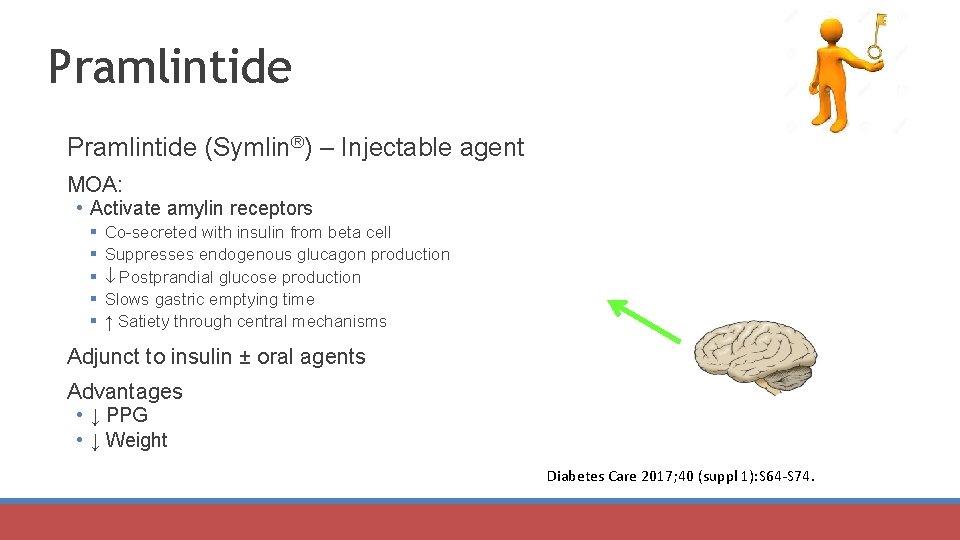
Pramlintide (Symlin®) – Injectable agent MOA: • Activate amylin receptors § § § Co-secreted with insulin from beta cell Suppresses endogenous glucagon production Postprandial glucose production Slows gastric emptying time ↑ Satiety through central mechanisms Adjunct to insulin ± oral agents Advantages • ↓ PPG • ↓ Weight Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
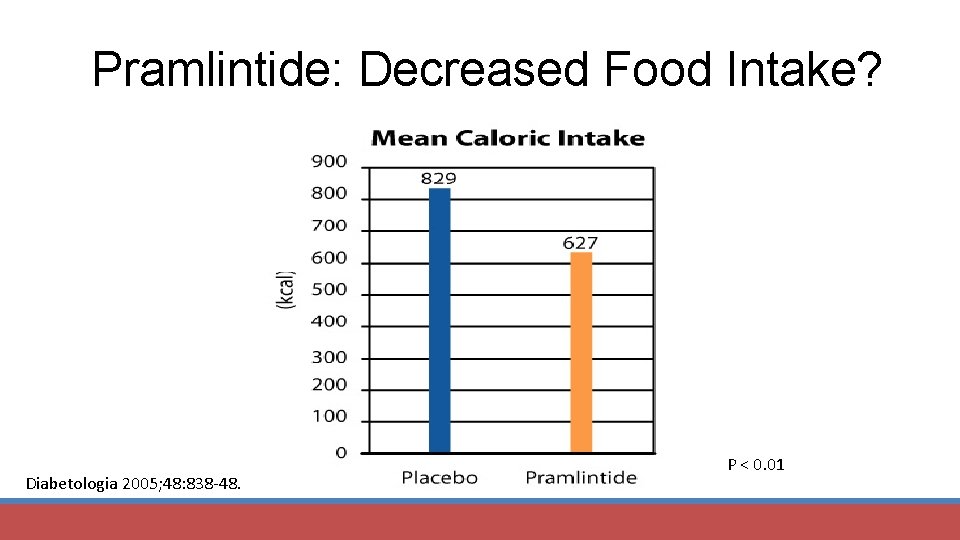
Pramlintide: Decreased Food Intake? Diabetologia 2005; 48: 838 -48. P < 0. 01

Pramlintide Side Effects/Limitations 1 • • Expensive injectable Frequent dosing ↑ Nausea, vomiting ↑ Hypoglycemia § Must prandial insulin dose by half Dose • Type 1: 15 mcg SC ac before major meals (250 calories/30 gm CHO) § Max: 60 mcg ac • Type 2: 60 mcg SC ac before major meals (250 calories/30 gm CHO) § Max: 120 mcg ac Effects • PPG (variable) ~ 60 -90 mg/d. L 2 • A 1 C – 0. 5%-1%1 1 Diabetes Care 2017; 40 (suppl 1): S 64 -S 74. 2 Drug Des Dev Ther 2009; 2: 203 -14.

Let’s Talk About The Incretin Effect
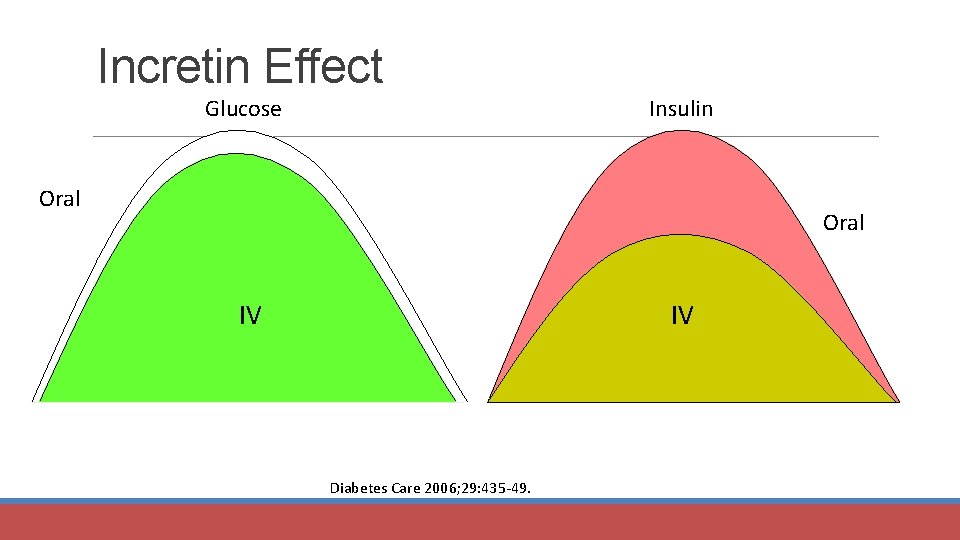
Incretin Effect Glucose Insulin Oral IV IV Diabetes Care 2006; 29: 435 -49.
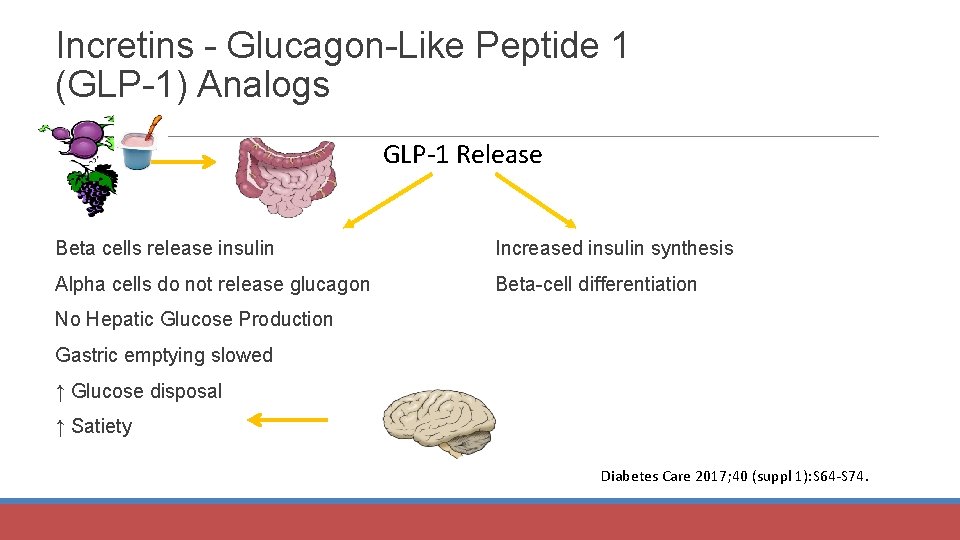
Incretins - Glucagon-Like Peptide 1 (GLP-1) Analogs GLP-1 Release Beta cells release insulin Increased insulin synthesis Alpha cells do not release glucagon Beta-cell differentiation No Hepatic Glucose Production Gastric emptying slowed ↑ Glucose disposal ↑ Satiety Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
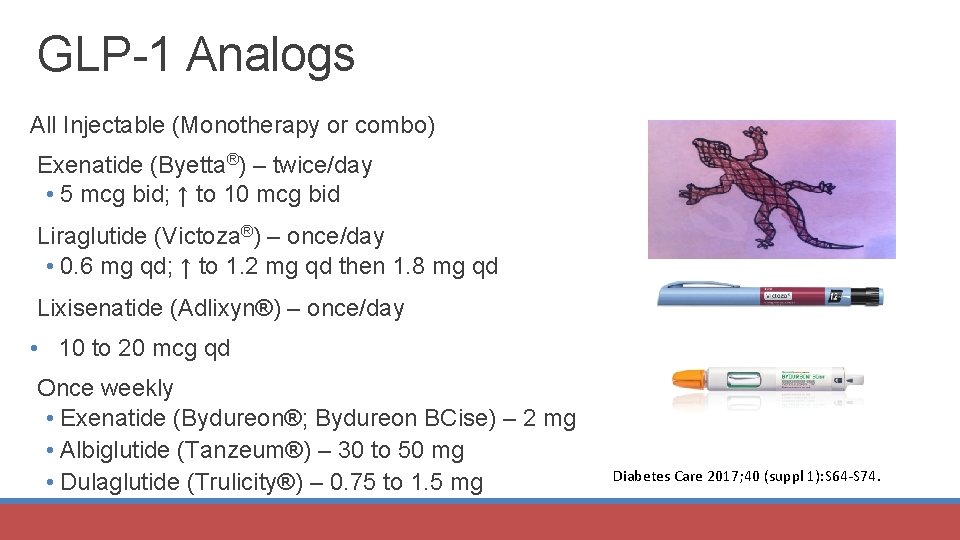
GLP-1 Analogs All Injectable (Monotherapy or combo) Exenatide (Byetta®) – twice/day • 5 mcg bid; ↑ to 10 mcg bid Liraglutide (Victoza®) – once/day • 0. 6 mg qd; ↑ to 1. 2 mg qd then 1. 8 mg qd Lixisenatide (Adlixyn®) – once/day • 10 to 20 mcg qd Once weekly • Exenatide (Bydureon®; Bydureon BCise) – 2 mg • Albiglutide (Tanzeum®) – 30 to 50 mg • Dulaglutide (Trulicity®) – 0. 75 to 1. 5 mg Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

GLP-1 Analogs MOA: • Activate GLP-1 receptors 1 Advantages • ↓ Postprandial glucose • Less hypoglycemia (↑ glucose-dependent insulin secretion) • Weight • Possible improved beta-cell mass/function • ↑ Satiety • Liraglutide – Aug 2017 FDA approved indication: �risk for myocardial infarction, stroke, and cardiovascular death in adults with type 2 diabetes with established cardiovascular disease 2 1 Diabetes Care 2017; 40 (suppl 1): S 64 -S 74. 2 http: //www. ajmc. com/newsroom/liraglutide-gains-new-indication-for-reducing-cv-event-risk

GLP-1 Analogs Side Effects/Limitations ($$$$) • • Injectable Nausea, vomiting Acute pancreatitis Renal dysfunction (exenatide) C-cell hyperplasia/medullary thyroid tumors (animal data) Drug interactions (↓ dose of sulfonylureas; �INR with warfarin) Durability Long-term safety? Effects • FPG 21 -33 mg/d. L; PPG (variable) up to 126 mg/d. L • A 1 C – 0. 8%-1. 6% • Weight loss is variable Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

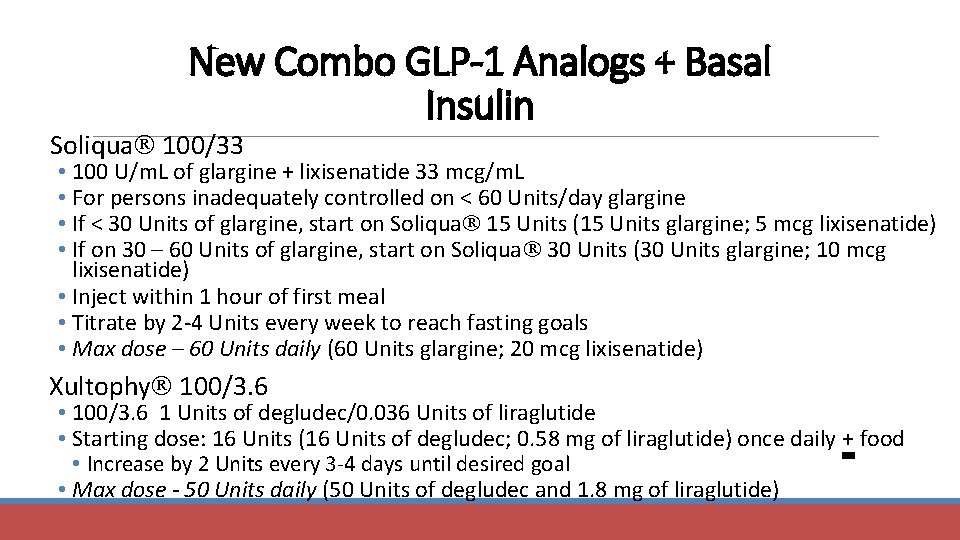
New Combo GLP-1 Analogs + Basal Insulin Soliqua 100/33 • 100 U/m. L of glargine + lixisenatide 33 mcg/m. L • For persons inadequately controlled on < 60 Units/day glargine • If < 30 Units of glargine, start on Soliqua 15 Units (15 Units glargine; 5 mcg lixisenatide) • If on 30 – 60 Units of glargine, start on Soliqua 30 Units (30 Units glargine; 10 mcg lixisenatide) • Inject within 1 hour of first meal • Titrate by 2 -4 Units every week to reach fasting goals • Max dose – 60 Units daily (60 Units glargine; 20 mcg lixisenatide) Xultophy 100/3. 6 • 100/3. 6 1 Units of degludec/0. 036 Units of liraglutide • Starting dose: 16 Units (16 Units of degludec; 0. 58 mg of liraglutide) once daily + food • Increase by 2 Units every 3 -4 days until desired goal • Max dose - 50 Units daily (50 Units of degludec and 1. 8 mg of liraglutide)
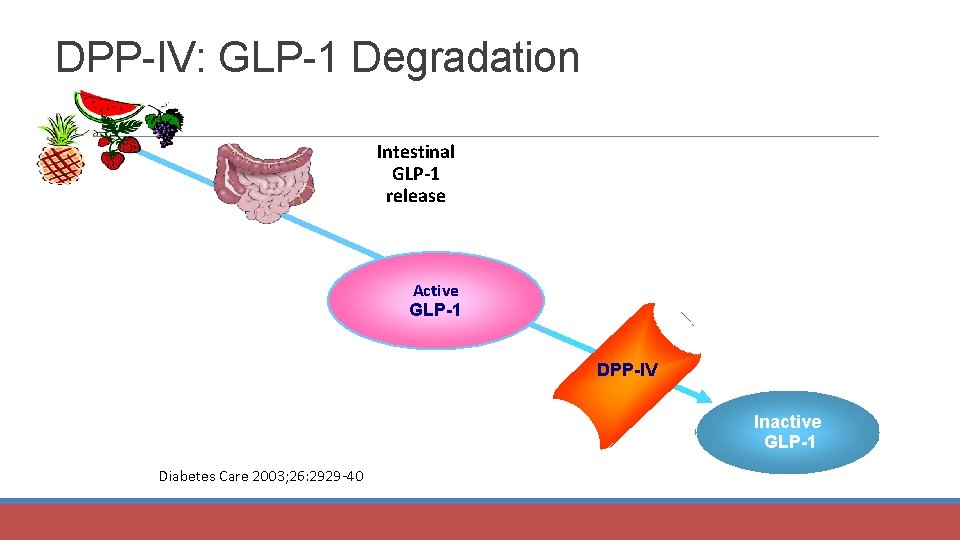
DPP-IV: GLP-1 Degradation Intestinal GLP-1 release Active GLP-1 DPP-IV Inactive GLP-1 Diabetes Care 2003; 26: 2929 -40.
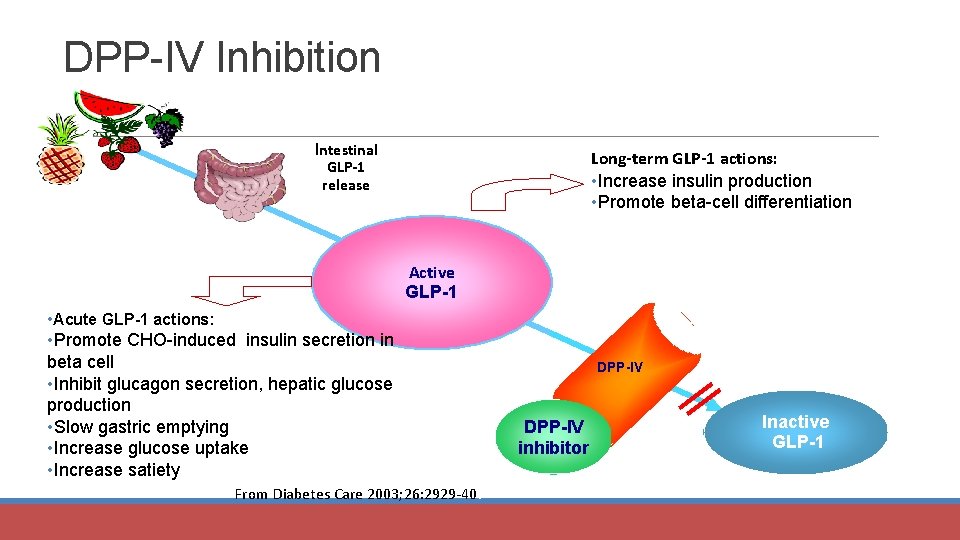
DPP-IV Inhibition Intestinal GLP-1 release Long-term GLP-1 actions: • Increase insulin production • Promote beta-cell differentiation Active GLP-1 • Acute GLP-1 actions: • Promote CHO-induced insulin secretion in beta cell • Inhibit glucagon secretion, hepatic glucose production • Slow gastric emptying • Increase glucose uptake • Increase satiety From Diabetes Care 2003; 26: 2929 -40. DPP-IV inhibitor Inactive GLP-1

DPP-IV Inhibitors Sitagliptin (Januvia®), saxagliptin (Onglyza®), linagliptin (Tradjenta®), alogliptin (Nesina® ) vildagliptin (in Europe) Mechanism of action • • • Inhibit DPP-4 activity; thus inhibit breakdown of GLP-1 and GIP Thus, levels of GLP-1 and GIP rise, especially in response to meals. § This inhibits glucagon (< GLP-1 analogs) § Stimulate endogenous insulin secretion when glucose is highest Because these agents increase only glucose-stimulated (by meals) insulin secretion, there is little risk of hypoglycemia. Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

DPP-IV Inhibitors Uses: Monotherapy or combination Advantages • ↓ Postprandial glucose • Less hypoglycemia (↑ glucose-dependent insulin secretion) • Well tolerated (better than sulfonylureas) • Weight neutral • Of benefit if A 1 C is close to goal Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.

DPP-IV Inhibitors Side Effects/Limitations ($$$$) • Allergic reactions (urticaria) • Acute pancreatitis • Headache • Infections (nasopharyngitis, URIs, UTIs) • Immune system dysfunction (T-cell activity) • Renal dysfunction (dose adjustment: sitagliptin, saxagliptin) • Drug interactions (CYP 3 A 4 issues: saxagliptin, linagliptin) • Durability? Long-term safety? (FDA: monitor for HF) Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
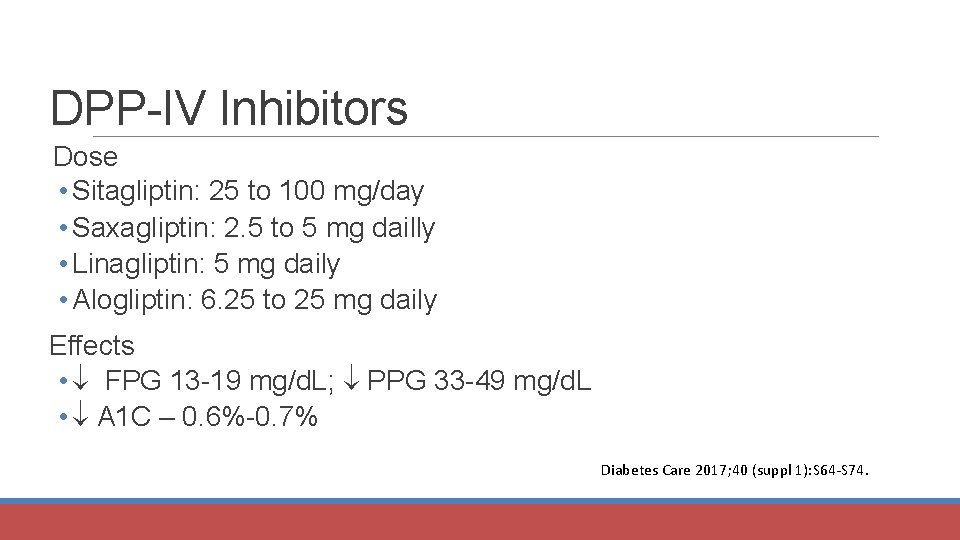
DPP-IV Inhibitors Dose • Sitagliptin: 25 to 100 mg/day • Saxagliptin: 2. 5 to 5 mg dailly • Linagliptin: 5 mg daily • Alogliptin: 6. 25 to 25 mg daily Effects • FPG 13 -19 mg/d. L; PPG 33 -49 mg/d. L • A 1 C – 0. 6%-0. 7% Diabetes Care 2017; 40 (suppl 1): S 64 -S 74.
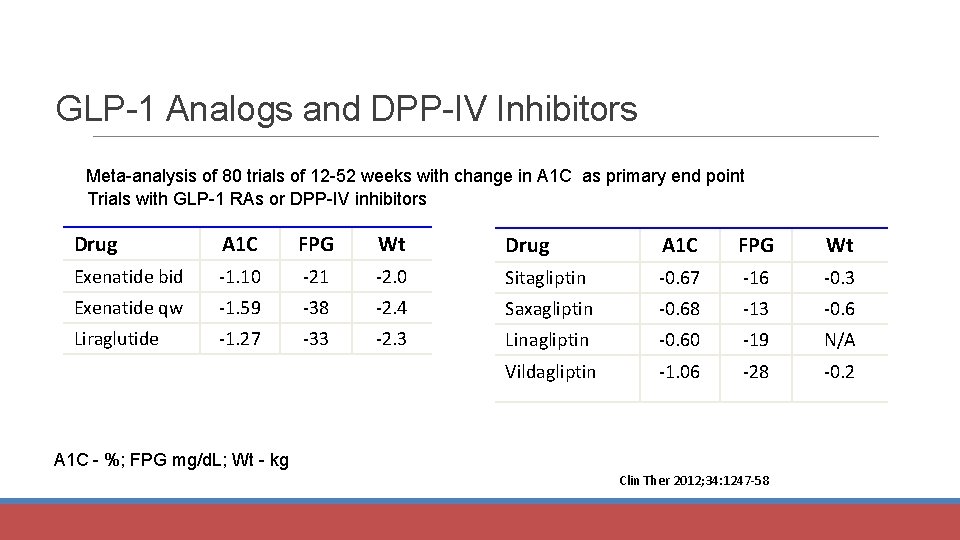
GLP-1 Analogs and DPP-IV Inhibitors Meta-analysis of 80 trials of 12 -52 weeks with change in A 1 C as primary end point Trials with GLP-1 RAs or DPP-IV inhibitors Drug A 1 C FPG Wt Exenatide bid -1. 10 -21 -2. 0 Sitagliptin -0. 67 -16 -0. 3 Exenatide qw -1. 59 -38 -2. 4 Saxagliptin -0. 68 -13 -0. 6 Liraglutide -1. 27 -33 -2. 3 Linagliptin -0. 60 -19 N/A Vildagliptin -1. 06 -28 -0. 2 A 1 C - %; FPG mg/d. L; Wt - kg Clin Ther 2012; 34: 1247 -58

Let’s Practice

L. H. HPI: LH is a 45 y/o obese female referred to DM Clinic by GYN for recurrent yeast infx and glycosuria on routine U/A. On 2 separate occasions, FPG 150 and 167. No polyphagia or polyuria, but more thirsty than usual. Has lethargy (afternoon naps). Gained 10 lb in last year. Other probs: RA, recurrent UTIs, FH + for DM. Labs: FPG – 147 mg/d. L; A 1 C – 9. 2% TGs – 400 mg/dl HDL 30 mg/d. L; LDL – cannot calculate LFTs – WNL; BUN 18; Cr 0. 9 mg/d. L GFR – 60 m. L/min Social hx: 1 ppd; occasional glass of wine

L. H. Candidate for metformin? Why? Action of metformin? Starting dose? A 1 C ↓? When to see expected efficacy?
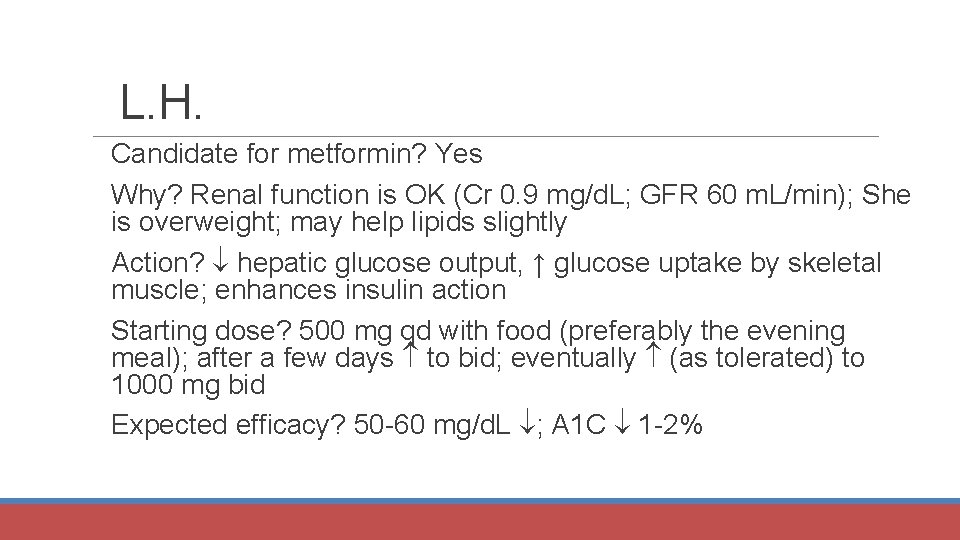
L. H. Candidate for metformin? Yes Why? Renal function is OK (Cr 0. 9 mg/d. L; GFR 60 m. L/min); She is overweight; may help lipids slightly Action? hepatic glucose output, ↑ glucose uptake by skeletal muscle; enhances insulin action Starting dose? 500 mg qd with food (preferably the evening meal); after a few days to bid; eventually (as tolerated) to 1000 mg bid Expected efficacy? 50 -60 mg/d. L ; A 1 C 1 -2%

L. H. Metformin ADRs? Drug interactions? Lab monitoring? Monitoring for lactic acidosis Counsel L. H. on use of metformin Overall monitoring?

L. H. Metformin ADRs? GI, anorexia, metallic taste Drug interactions? ETOH/radiocontrast media are major; minor interactions with cimetidine Lab monitoring? Cr, B 12 Monitoring for lactic acidosis (binge ETOH, serious dehydration, radiocontrast media procedures, surgery, hypoxic states; lactate and pyruvate levels) Counsel L. H. on use of metformin – Take with food; do not binge drink ETOH; monitor for signs/symptoms of lactic acidosis (SOB, myalgias, palpitations, etc. ) Monitoring: BG, A 1 C, renal function; lactic acidosis

L. H. Candidate for SU? If a candidate, select an oral SU for treatment Med and dose? Action? Expected efficacy? ADRs? Drug ixns? Lab monitoring? Counseling

L. H. Candidate? Yes Med and dose? Glipizide, 2. 5 mg qd (can use others) Action? Releases insulin from beta cell; e. g. , ↑ insulin secretion Expected efficacy? 50 -60 mg/d. L ; A 1 C 1 -2% ADRs? Hypoglycemia, weight gain, GI upset, photosensitivity, SIADH possible, disulfiram rxns
L. H. Drug interactions? • Additive effects with ETOH, sulfonamides, salicylates • Antagonistic effects of steroids, antipsychotics, phenytoin, lithium, thiazides, niacin Monitoring – BG, hypoglycemia, A 1 C Counseling L. H. on use of SU – • Take daily with food • May cause hypoglycemia so do not delay/skip meals • Wear sunscreen and sunglasses

L. H. Candidate for TZD? Action Starting dose Expected efficacy ADRs

L. H. Candidate for TZD? Yes; Pio or rosi Action – ↑ insulin sensitivity; site of action: muscle, liver Starting dose – pio: 15 mg; rosi: 2 mg Expected efficacy – A 1 C ↓ 0. 5% to 1. 4% ADRs – ↑ weight; edema; HF; ↑ LFTs; fractures; macular edema
L. H. Drug interactions • ↑ LFTs with liver toxic meds • + insulin = edema • Pio induces CYP 3 A 4 • Gemfibrozil ↑ AUC of pio/rosi • Rifampin ↓ AUC TZD Lab monitoring – LFTs? BG, A 1 C Counsel L. H. on use – Monitor weight, monitor for edema, onset of effect takes several weeks (12 wks); Monitor for osteoporosis; Caution in females of childbearing age

L. H. Candidate for acarbose or miglitol? MOA? Starting dose? Expected A 1 C decrease? ADRs? Drug interactions? Lab monitoring? Counsel L. H. on use of these agents
L. H. Candidate for acarbose or miglitol? Yes Action – Slowed intestinal CHO absorption Starting dose – 25 mg with food; titrate slowly Expected efficacy – A 1 C ↓ 0. 5% to 0. 8% ADRs – GI (gas, bloating, diarrhea, borborygmus) Drug interactions – May bind some meds; + short-acting/rapid insulin = hypoglycemia Lab monitoring – LFTs? BG, A 1 C Counsel L. H. on use – titrate slowly; GI side effects; if used with SU/insulin, use glucose tablets for hypoglycemia Sx; max response: 6 months Advantage of AGIs? ↓ PPG

L. H. Candidate for Colesevelam? Bromocriptine? Action? Starting dose? Expected efficacy? ADRs? Drug interactions? Lab monitoring? Counsel L. H. on use of these agents

L. H. Candidate for SGLT-2 Inhibitors? Action? Expected efficacy? ADRs? Drug interactions? Lab monitoring? Counsel L. H. on use of these agents
L. H. Candidate for SGLT-2 Inhibitors? Yes Action? Inhibit urinary glucose reabsorption; glucose excretion Expected efficacy? – 1% in A 1 C; FPG 25 mg/d. L ADRs? Infections, hypotension, euglycemic KA, fractures, bladder/breast CA (dapagliflozin) amputations (canaglifloxin) Drug interactions? - BP with BP meds; K with ACEI (canagliflozin), NSAIDs ( dapagliflozin SDCs) Lab monitoring? – Only BG/A 1 C Counsel L. H. on use of these agents – you may lose weight, BP may ; possible infections; stay hydrated; do not stop insulin if you are on it

Antihyperglycemic therapy in type 2 diabetes: general recommendations. Dual Therapy: A 1 C > 9% Combo Injectable: A 1 C > 10% or BG > 300 mg/d. L Metformin Intolerance? American Diabetes Association Diabetes Care 2017; 40: S 64 -S 74 © 2017 by American Diabetes Association

Things to Ponder

L. H. HPI: LH is a 45 y/o obese female referred to DM Clinic by GYN for recurrent yeast infx and glycosuria on routine U/A. On 2 separate occasions, FPG 150 and 167. No polyphagia or polyuria, but more thirsty than usual. Has lethargy (afternoon naps). Gained 10 lb in last year. Other probs: RA, recurrent UTIs, FH + for DM. Labs: FPG – 147 mg/d. L; A 1 C – 8. 9% TGs – 430 mg/dl HDL 30 mg/d. L; LDL – cannot calculate LFTs – WNL; BUN 18; Cr 0. 9 mg/d. L; GFR 60 m. L/min Social hx: 1 ppd; occasional glass of wine Starting agent?

L. H. After optimizing the medication you started LH on, her A 1 C goes down to 6. 9%. After 2 years her A 1 C goes back up and is now 9. 5%. What would you do?

L. H. After a few years you add a third agent (and then a fourth or even a fifth agent) to LH’s regimen but now her glucose has escalated and her A 1 C is now 11. 2%. What would you do?
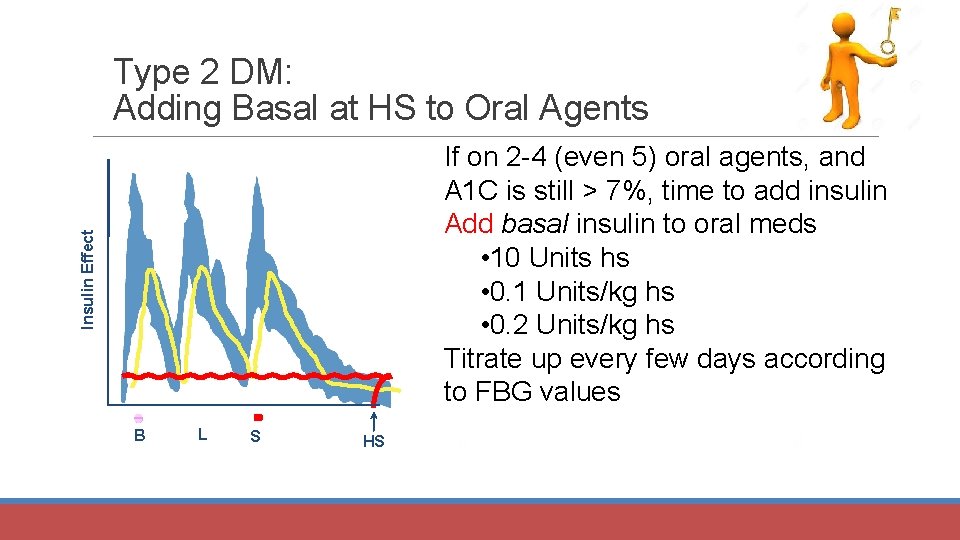
Type 2 DM: Adding Basal at HS to Oral Agents Insulin Effect If on 2 -4 (even 5) oral agents, and A 1 C is still > 7%, time to add insulin Add basal insulin to oral meds • 10 Units hs • 0. 1 Units/kg hs • 0. 2 Units/kg hs Titrate up every few days according to FBG values B L S HS B B
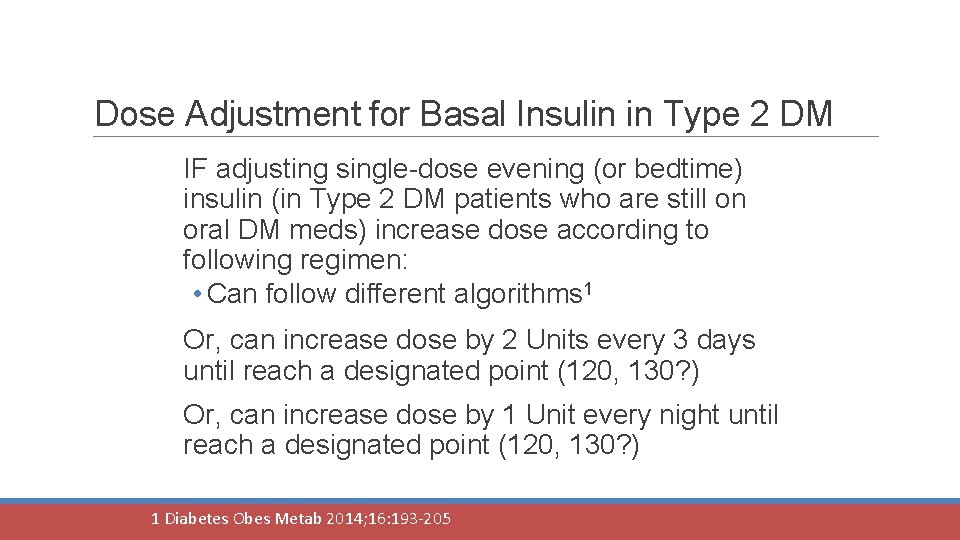
Dose Adjustment for Basal Insulin in Type 2 DM IF adjusting single-dose evening (or bedtime) insulin (in Type 2 DM patients who are still on oral DM meds) increase dose according to following regimen: • Can follow different algorithms 1 Or, can increase dose by 2 Units every 3 days until reach a designated point (120, 130? ) Or, can increase dose by 1 Unit every night until reach a designated point (120, 130? ) 1 Diabetes Obes Metab 2014; 16: 193 -205


Combination injectable therapy for type 2 diabetes. American Diabetes Association Dia Care 2017; 40: S 64 -S 74 © 2017 by American Diabetes Association
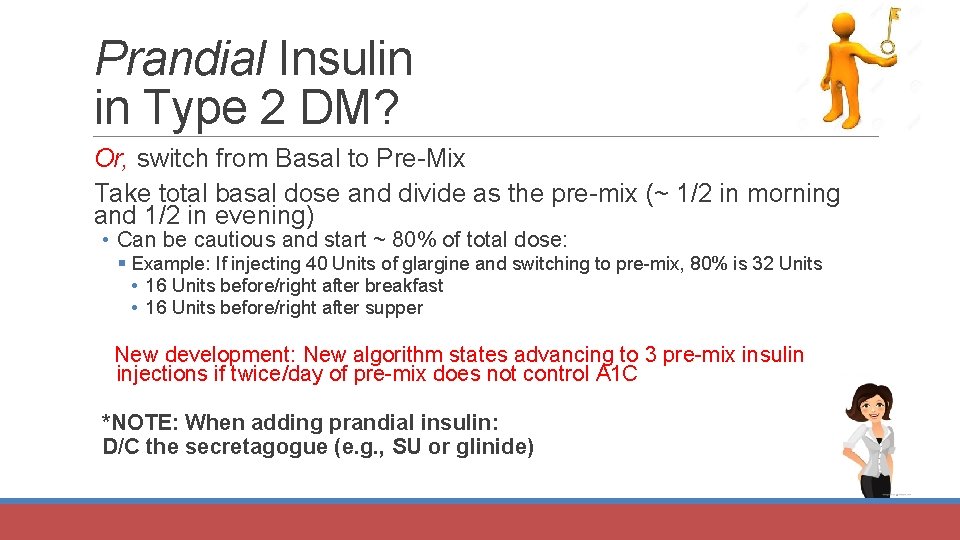
Prandial Insulin in Type 2 DM? Or, switch from Basal to Pre-Mix Take total basal dose and divide as the pre-mix (~ 1/2 in morning and 1/2 in evening) • Can be cautious and start ~ 80% of total dose: § Example: If injecting 40 Units of glargine and switching to pre-mix, 80% is 32 Units • 16 Units before/right after breakfast • 16 Units before/right after supper New development: New algorithm states advancing to 3 pre-mix insulin injections if twice/day of pre-mix does not control A 1 C *NOTE: When adding prandial insulin: D/C the secretagogue (e. g. , SU or glinide)

Prandial Insulin in Type 2 DM? Or go directly to Basal/Bolus ½ of the total daily dose will be basal ½ of the total daily dose will be bolus • Split up among the major meals *REMEMBER: When adding prandial insulin, D/C the secretagogue (e. g. , SU or glinide)

Conclusions • Etiology of DM is complex • Many pathophysiologic changes occur in DM • Insulin is available to treat T 1 and T 2 DM • Pramlintide is available to treat T 1 and T 2 DM • Several oral medications are available to treat T 2 DM • Treatment should target the pathophysiologic changes
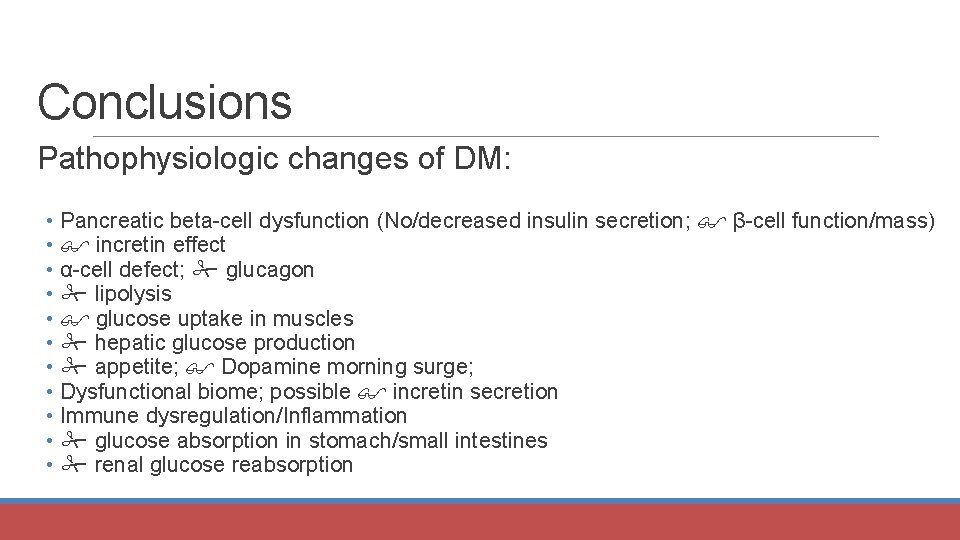
Conclusions Pathophysiologic changes of DM: • Pancreatic beta-cell dysfunction (No/decreased insulin secretion; β-cell function/mass) • incretin effect • α-cell defect; glucagon • lipolysis • glucose uptake in muscles • hepatic glucose production • appetite; Dopamine morning surge; • Dysfunctional biome; possible incretin secretion • Immune dysregulation/Inflammation • glucose absorption in stomach/small intestines • renal glucose reabsorption

QUESTIONS?
- Slides: 85