Nomenclature Working through Naming Compounds and Writing Formulas




![Q 6: The formula for tetrachlorferrate(III) is 1) [Fe. Cl 3]+ 2) [Fe. Cl Q 6: The formula for tetrachlorferrate(III) is 1) [Fe. Cl 3]+ 2) [Fe. Cl](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-7.jpg)
![Q 7: The formula of hexaquacobalt(II) is 1) [Co(H 2 O)4]22) [Co(H 2 O)6]23) Q 7: The formula of hexaquacobalt(II) is 1) [Co(H 2 O)4]22) [Co(H 2 O)6]23)](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-8.jpg)
![Q 9: The name of the following complex is: [Co(NH 3)5 Cl]SO 4 1) Q 9: The name of the following complex is: [Co(NH 3)5 Cl]SO 4 1)](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-10.jpg)
![Q 10: The name of the following complex is: [Co(en)2 Cl 2]Cl 1) bis(ethylenediamine)dichlorocobalt(II) Q 10: The name of the following complex is: [Co(en)2 Cl 2]Cl 1) bis(ethylenediamine)dichlorocobalt(II)](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-11.jpg)

- Slides: 13

Nomenclature Working through Naming Compounds and Writing Formulas with Examples Created by James Kirby, Quinnipiac University (james. kirby@quinnipiac. edu) and posted on VIPEr (www. ionicviper. org) on July 2, 2015. Copyright James Kirby 2015. The work is licensed under the Creative Commons Attribution Noncommercial Share Alike License. To view a copy of this license, visit http: //creativecommons. org/about/license/.

Q 1: The formula of the ionic compound formed by sodium, Na (Group 1 or IA), and oxygen, O (Group 16 or VIA), is 1) Na. O 2 3) Na 2 O 4) Na 2 O 2

Q 2: Na 2 O is 1) disodium monoxide 2) disodium oxide 3) sodium monoxide 4) sodium oxide 5) sodium(II) oxide

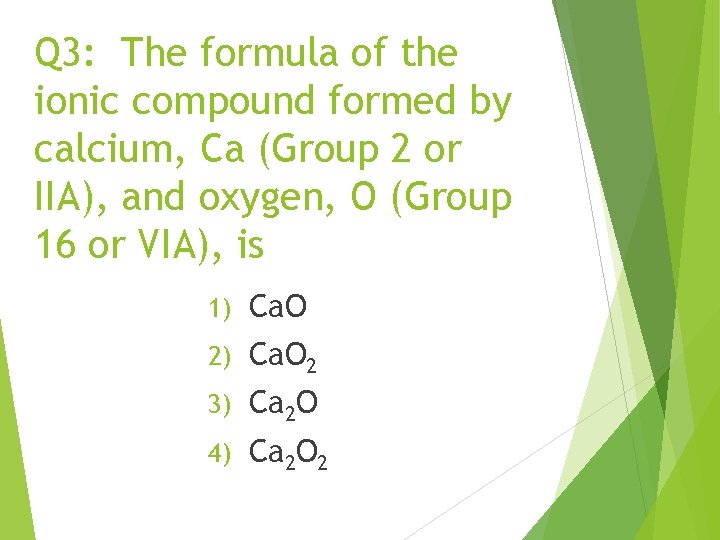
Q 3: The formula of the ionic compound formed by calcium, Ca (Group 2 or IIA), and oxygen, O (Group 16 or VIA), is 1) Ca. O 2 3) Ca 2 O 4) Ca 2 O 2

Q 4: Ca. O is 1) calcium monoxide 2) calcium oxide 3) calcium(II) monoxide 4) calcium(II) oxide 5) calcium oxide(2 -)

Q 5: Co. O is 1) cobalt monoxide 2) cobalt oxide 3) cobalt(II) monoxide 4) cobalt(II) oxide 5) cobalt(III) monoxide 6) cobalt(III) oxide 7) monocobalt monoxide
![Q 6 The formula for tetrachlorferrateIII is 1 Fe Cl 3 2 Fe Cl Q 6: The formula for tetrachlorferrate(III) is 1) [Fe. Cl 3]+ 2) [Fe. Cl](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-7.jpg)
Q 6: The formula for tetrachlorferrate(III) is 1) [Fe. Cl 3]+ 2) [Fe. Cl 3]3 - 3) [Fe. Cl 3]3+ 4) [Fe. Cl 4]- 5) [Fe. Cl 4]3 -
![Q 7 The formula of hexaquacobaltII is 1 CoH 2 O422 CoH 2 O623 Q 7: The formula of hexaquacobalt(II) is 1) [Co(H 2 O)4]22) [Co(H 2 O)6]23)](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-8.jpg)
Q 7: The formula of hexaquacobalt(II) is 1) [Co(H 2 O)4]22) [Co(H 2 O)6]23) [Co(H 2 O)4]2+ 4) [Co(H 2 O)6]2+ 5) [Co(H 2 O)4]3+ 6) [Co(H 2 O)6]3+

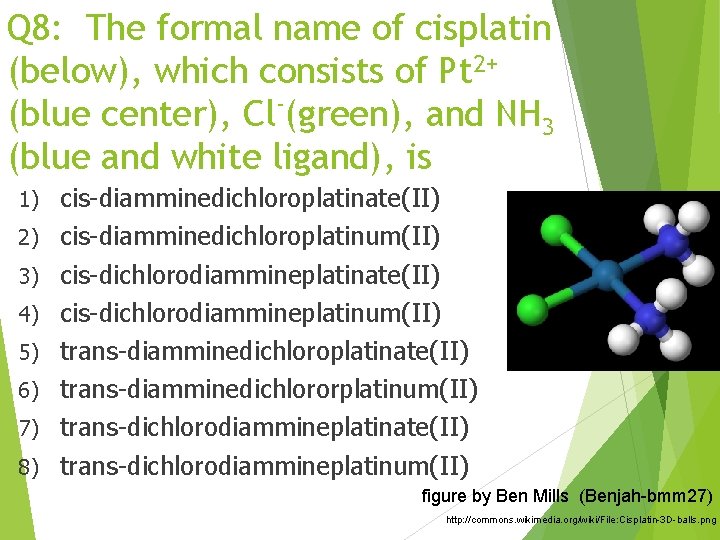
Q 8: The formal name of cisplatin (below), which consists of Pt 2+ (blue center), Cl (green), and NH 3 (blue and white ligand), is 1) 2) 3) 4) 5) 6) 7) 8) cis-diamminedichloroplatinate(II) cis-diamminedichloroplatinum(II) cis-dichlorodiammineplatinate(II) cis-dichlorodiammineplatinum(II) trans-diamminedichloroplatinate(II) trans-diamminedichlororplatinum(II) trans-dichlorodiammineplatinate(II) trans-dichlorodiammineplatinum(II) figure by Ben Mills (Benjah-bmm 27) http: //commons. wikimedia. org/wiki/File: Cisplatin-3 D-balls. png
![Q 9 The name of the following complex is CoNH 35 ClSO 4 1 Q 9: The name of the following complex is: [Co(NH 3)5 Cl]SO 4 1)](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-10.jpg)
Q 9: The name of the following complex is: [Co(NH 3)5 Cl]SO 4 1) chloropentamminecobalt(II) sulfate 2) chloropentamminecobalt(III) sulfate 3) chloropentamminesulfatocobalt 4) chloropentammoniasulfatocobalt 5) pentamminechlorocobalt(II) sulfate 6) pentamminechlorocobalt(III) sulfate 7) pentammoniachlorocobalt(III) sulfate
![Q 10 The name of the following complex is Coen2 Cl 2Cl 1 bisethylenediaminedichlorocobaltII Q 10: The name of the following complex is: [Co(en)2 Cl 2]Cl 1) bis(ethylenediamine)dichlorocobalt(II)](https://slidetodoc.com/presentation_image_h/0be4044aef7c6c0d38ef97330260f42e/image-11.jpg)
Q 10: The name of the following complex is: [Co(en)2 Cl 2]Cl 1) bis(ethylenediamine)dichlorocobalt(II) chloride 2) bis(ethylenediamine)dichlorocobalt(III) chloride 3) dichlorodiethylenediaminecobalt(II) chloride 4) dichlorodbis(ethylenediamine)cobalt(II) chloride 5) dichlorodbis(ethylenediamine)cobalt(III) chloride 6) dichlorodiethylenediaminecobaltate(II) chloride 7) dichlorodiethylenediaminecobaltate(III) chloride 8) dichlorobis(ethylenediamine)cobaltate(II) chloride 9) dichlorobis(ethylenediamine)cobaltate(III) chloride

Q 11: The formula for bis(μ-hydroxo)bis(tetraamminecobalt(II)) is 1) (NH 3)4 Co(OH)2 2) (NH 4)2 Co(OH)2 3) (NH 3)4 Co(OH)2 Co(NH 3)4 4) [(NH 3)4 Co(OH)2 Co(NH 3)4]2+ 5) (NH 4)2 Co(OH)2 Co(NH 4)2

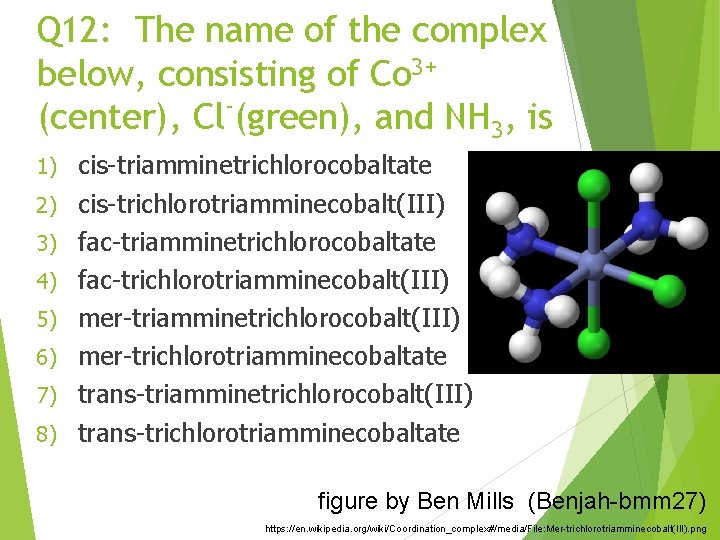
Q 12: The name of the complex below, consisting of Co 3+ (center), Cl (green), and NH 3, is 1) 2) 3) 4) 5) 6) 7) 8) cis-triamminetrichlorocobaltate cis-trichlorotriamminecobalt(III) fac-triamminetrichlorocobaltate fac-trichlorotriamminecobalt(III) mer-triamminetrichlorocobalt(III) mer-trichlorotriamminecobaltate trans-triamminetrichlorocobalt(III) trans-trichlorotriamminecobaltate figure by Ben Mills (Benjah-bmm 27) https: //en. wikipedia. org/wiki/Coordination_complex#/media/File: Mer-trichlorotriamminecobalt(III). png