Nomenclature Table of Contents Nomenclature Binary Compounds Metal

Nomenclature

Table of Contents ‘Nomenclature’ Binary Compounds - Metal (fixed oxidation) + Nonmetal Criss-Cross Rule Binary Compounds - Metal (variable oxidation) + Nonmetal Binary Compounds - Nonmetal + Nonmetal Ternary Compounds Binary Hydrogen Compounds Meaning of Suffixes Empirical Formulas Subscripts, Superscripts, and Coefficients Centrum Multivitamin Polyatomic Ions
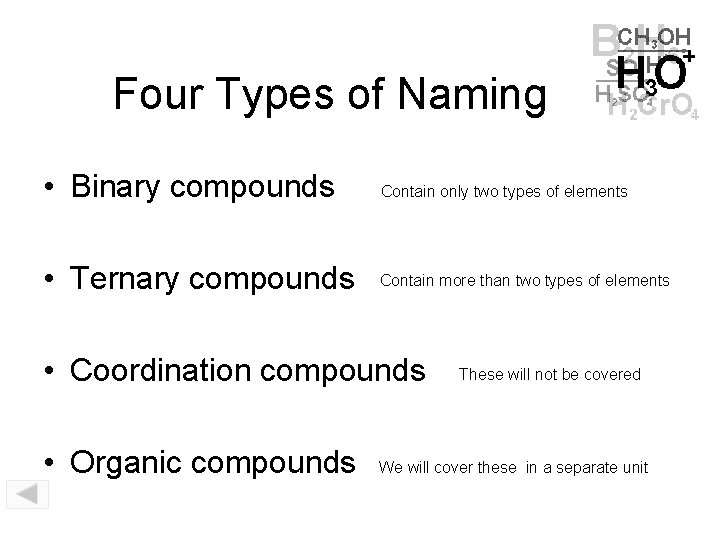
Four Types of Naming • Binary compounds Contain only two types of elements • Ternary compounds Contain more than two types of elements • Coordination compounds • Organic compounds These will not be covered We will cover these in a separate unit

Binary Compounds Metals (fixed oxidation) + Nonmetals
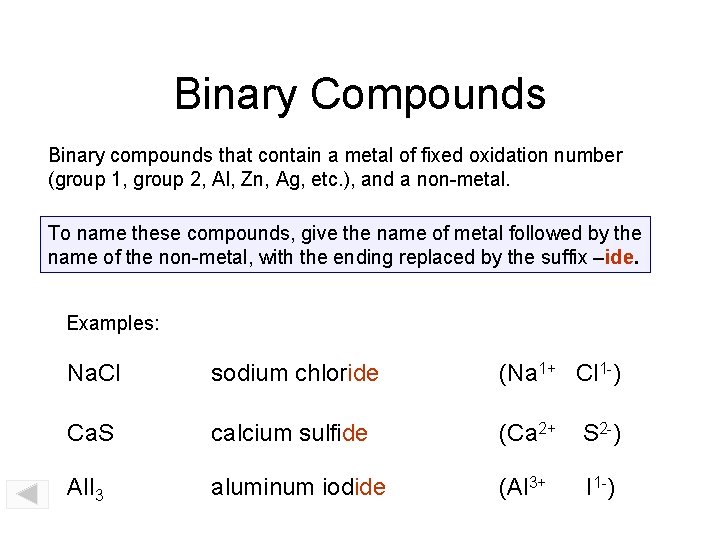
Binary Compounds Binary compounds that contain a metal of fixed oxidation number (group 1, group 2, Al, Zn, Ag, etc. ), and a non-metal. To name these compounds, give the name of metal followed by the name of the non-metal, with the ending replaced by the suffix –ide. Examples: Na. Cl sodium chloride (Na 1+ Cl 1 -) Ca. S calcium sulfide (Ca 2+ S 2 -) Al. I 3 aluminum iodide (Al 3+ I 1 -)
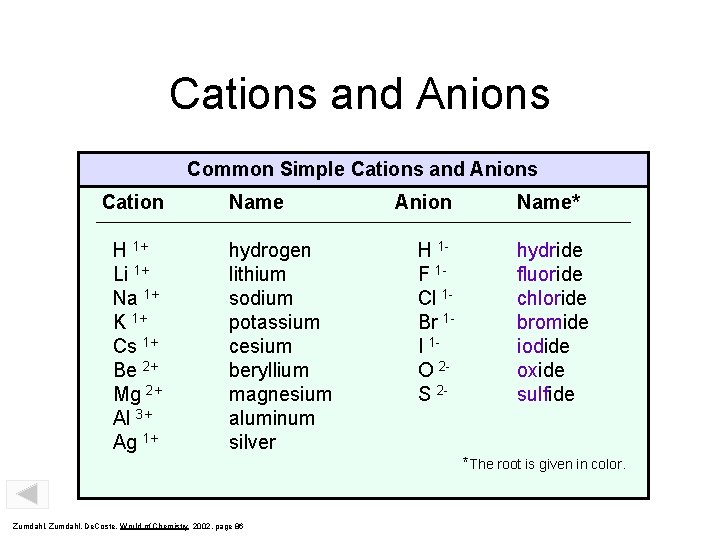
Cations and Anions Common Simple Cations and Anions Cation H 1+ Li 1+ Na 1+ K 1+ Cs 1+ Be 2+ Mg 2+ Al 3+ Ag 1+ Name hydrogen lithium sodium potassium cesium beryllium magnesium aluminum silver Anion H 1 F 1 Cl 1 Br 1 I 1 O 2 S 2 - Name* hydride fluoride chloride bromide iodide oxide sulfide *The root is given in color. Zumdahl, De. Coste, World of Chemistry 2002, page 86
Ionic Binary Compounds: Single-Charge Cations Keys
- Slides: 7