Nomenclature Scientific Naming System What is the difference
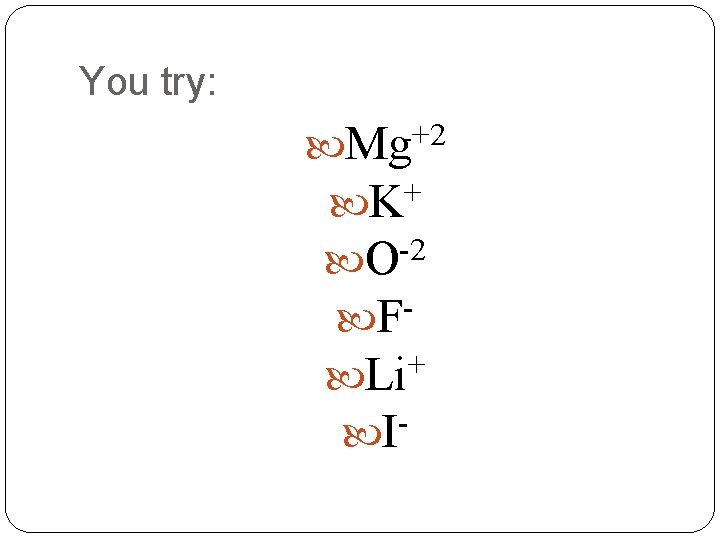






- Slides: 21

Nomenclature Scientific Naming System

What is the difference between an element and a compound?

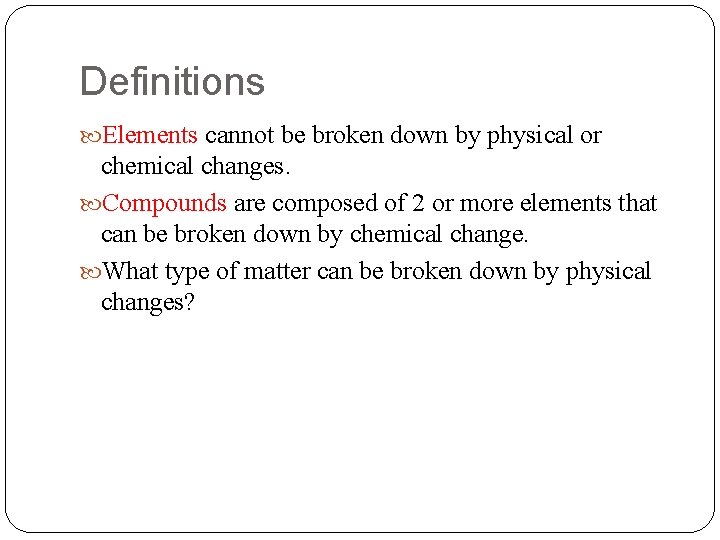
Definitions Elements cannot be broken down by physical or chemical changes. Compounds are composed of 2 or more elements that can be broken down by chemical change. What type of matter can be broken down by physical changes?

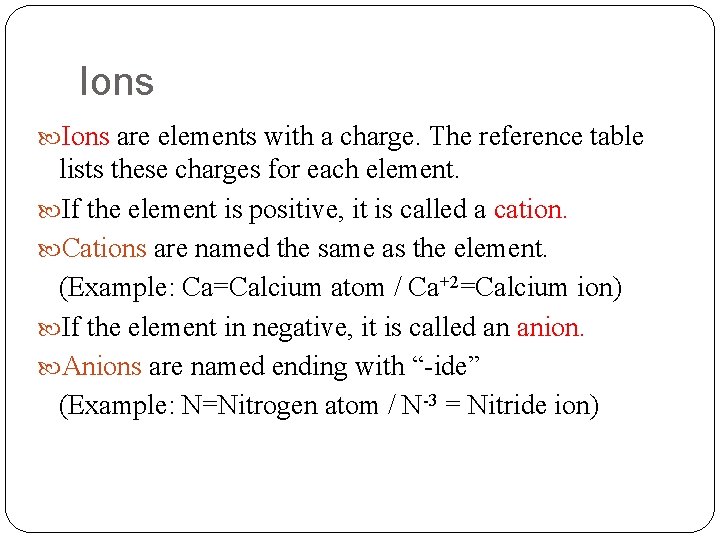
Ions are elements with a charge. The reference table lists these charges for each element. If the element is positive, it is called a cation. Cations are named the same as the element. (Example: Ca=Calcium atom / Ca+2=Calcium ion) If the element in negative, it is called an anion. Anions are named ending with “-ide” (Example: N=Nitrogen atom / N-3 = Nitride ion)
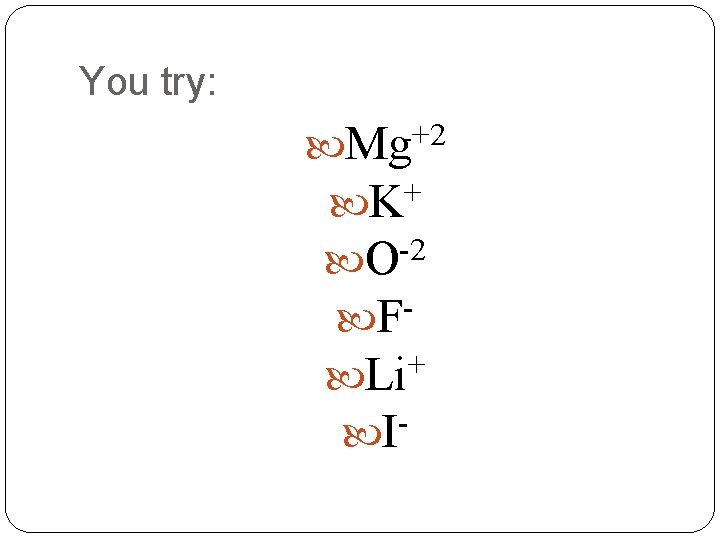
You try: Mg+2 + K O-2 F + Li I-

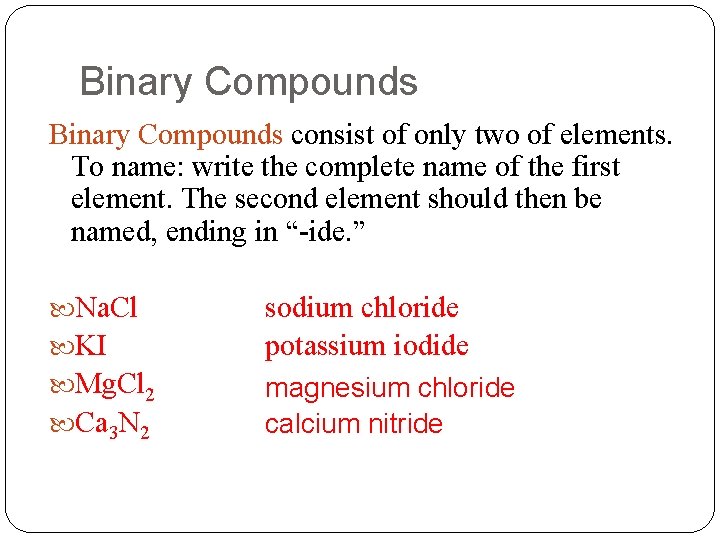
Binary Compounds consist of only two of elements. To name: write the complete name of the first element. The second element should then be named, ending in “-ide. ” Na. Cl KI Mg. Cl 2 Ca 3 N 2 sodium chloride potassium iodide magnesium chloride calcium nitride

You try… Li 3 P Al 2 S 3 Sr. Br 2 Rb 2 O Ba. Se Cs. I

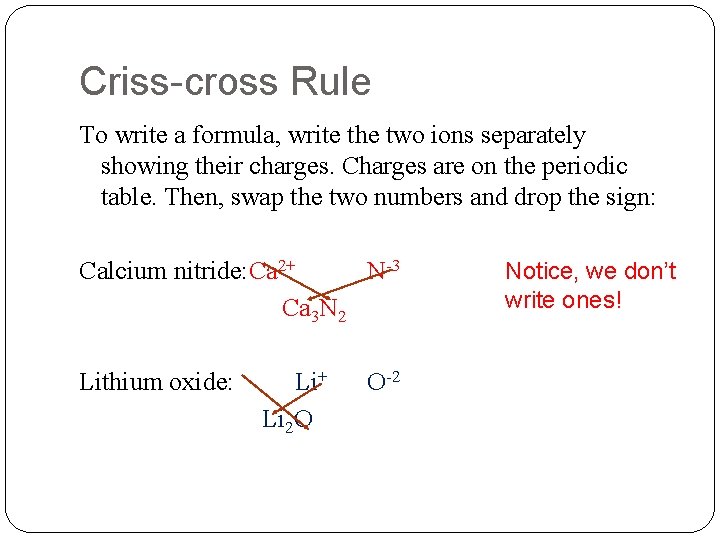
Criss-cross Rule To write a formula, write the two ions separately showing their charges. Charges are on the periodic table. Then, swap the two numbers and drop the sign: Calcium nitride: Ca 2+ N-3 Ca 3 N 2 Lithium oxide: Li+ Li 2 O O-2 Notice, we don’t write ones!

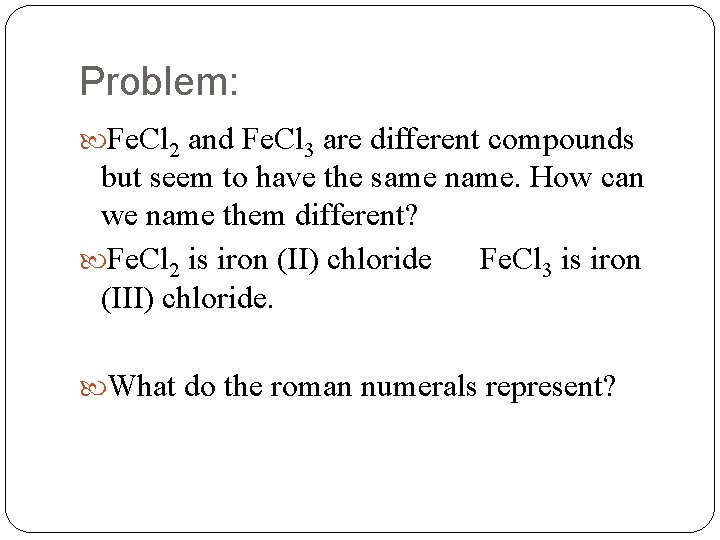
Problem: Fe. Cl 2 and Fe. Cl 3 are different compounds but seem to have the same name. How can we name them different? Fe. Cl 2 is iron (II) chloride Fe. Cl 3 is iron (III) chloride. What do the roman numerals represent?

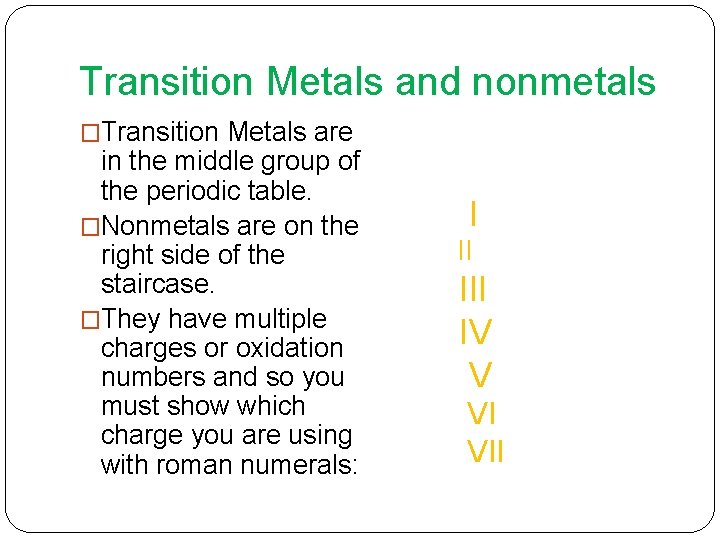
Transition Metals and nonmetals �Transition Metals are in the middle group of the periodic table. �Nonmetals are on the right side of the staircase. �They have multiple charges or oxidation numbers and so you must show which charge you are using with roman numerals: I II IV V VI VII

Try these… Fe. Cl 2 Cu. F Zn. O N 2 O 3 SO 4 PCl 3 CH 4

Careful: This rule doesn’t ALWAYS work for cations. Find the anion’s charge and equalize that with the cation’s charge as a check.

Try these… Potassium iodide Magnesium chloride Aluminum sulfide Hydrogen oxide Barium selenide Cesium phosphide Strontium phosphide Copper (II) flouride Iron (III) telluride

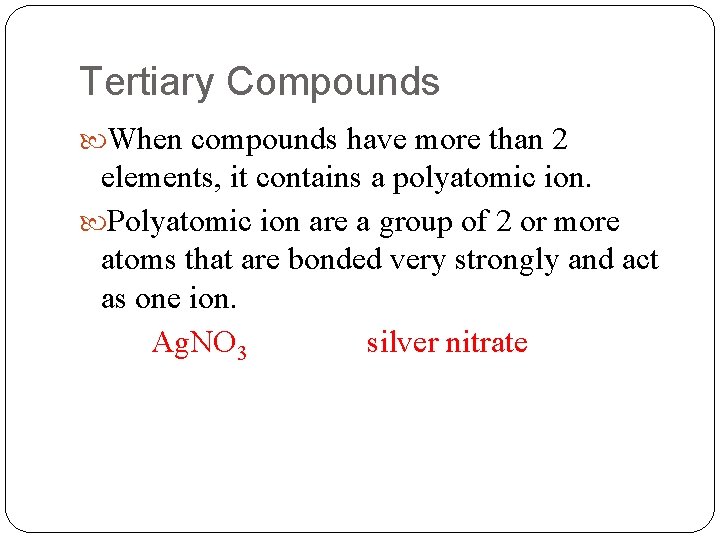
Tertiary Compounds When compounds have more than 2 elements, it contains a polyatomic ion. Polyatomic ion are a group of 2 or more atoms that are bonded very strongly and act as one ion. Ag. NO 3 silver nitrate

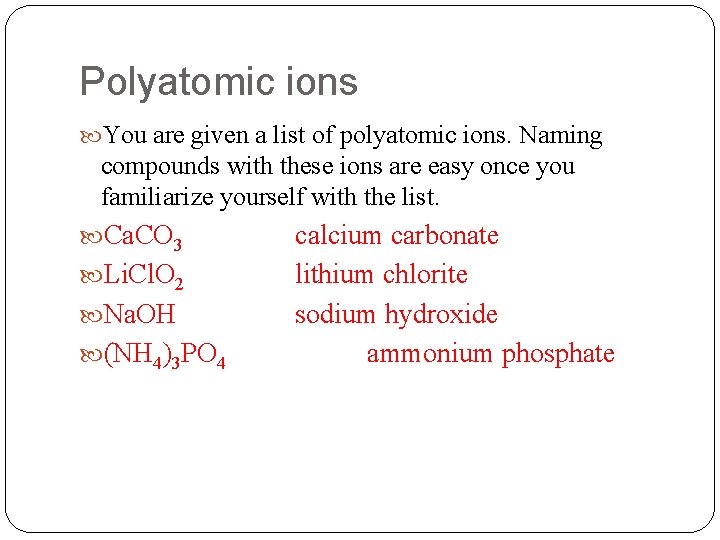
Polyatomic ions You are given a list of polyatomic ions. Naming compounds with these ions are easy once you familiarize yourself with the list. Ca. CO 3 Li. Cl. O 2 Na. OH (NH 4)3 PO 4 calcium carbonate lithium chlorite sodium hydroxide ammonium phosphate

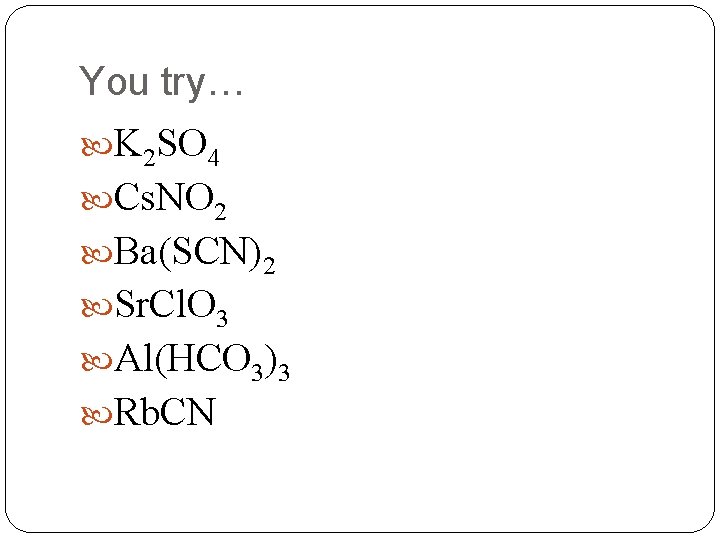
You try… K 2 SO 4 Cs. NO 2 Ba(SCN)2 Sr. Cl. O 3 Al(HCO 3)3 Rb. CN

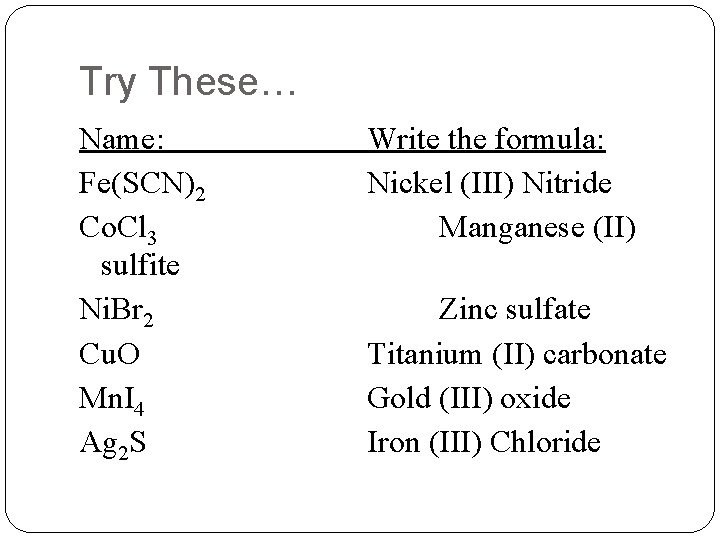
Try These… Name: Fe(SCN)2 Co. Cl 3 sulfite Ni. Br 2 Cu. O Mn. I 4 Ag 2 S Write the formula: Nickel (III) Nitride Manganese (II) Zinc sulfate Titanium (II) carbonate Gold (III) oxide Iron (III) Chloride

Game http: //www. sciencegeek. net/APchemistry/APtaters/direct ory. shtml

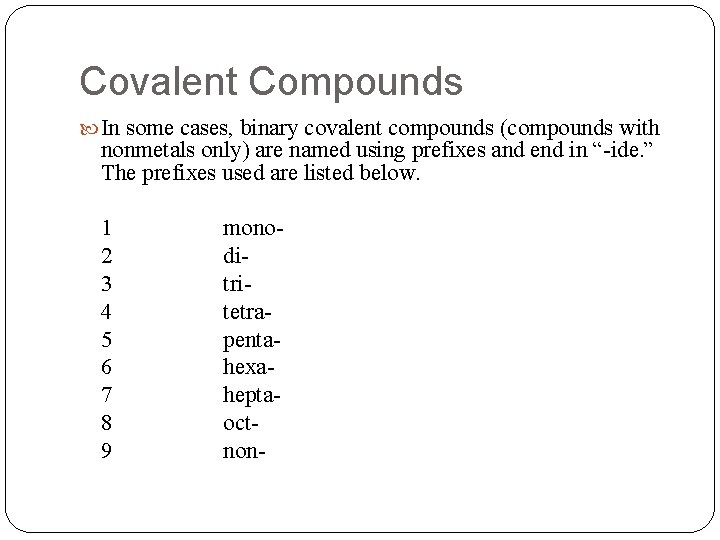
Covalent Compounds In some cases, binary covalent compounds (compounds with nonmetals only) are named using prefixes and end in “-ide. ” The prefixes used are listed below. 1 2 3 4 5 6 7 8 9 monoditritetrapentahexaheptaoctnon-

Name or write the formula: NO H 2 O PCl 5 Cl 2 O 7 Carbon tetrachloride Phosphorous tribromide Silicon dioxide
