Nomenclature Rules for Identifying Simple Chemical Compounds Naming





















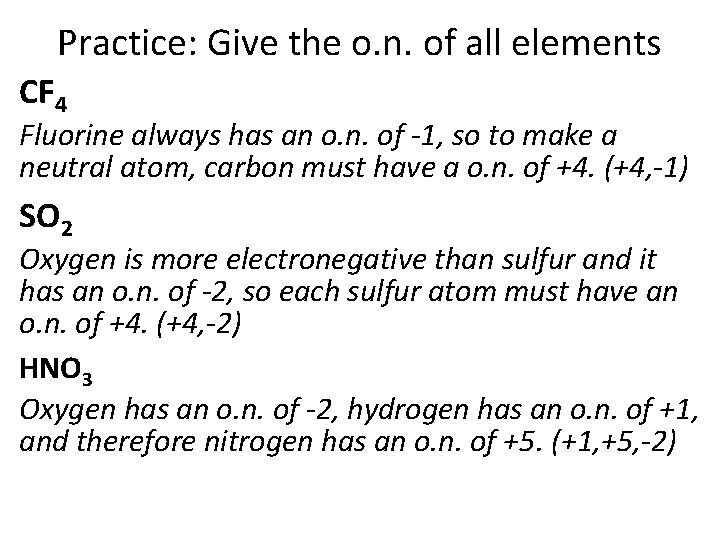
- Slides: 52

Nomenclature Rules for Identifying Simple Chemical Compounds: Naming and Formulae

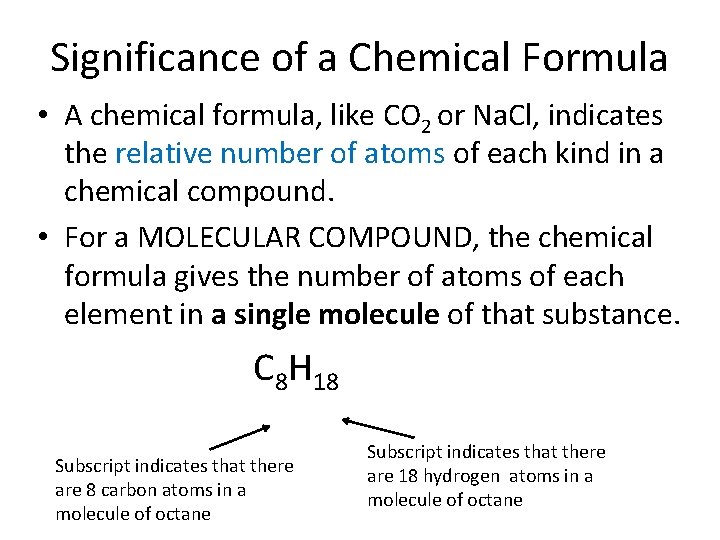
Significance of a Chemical Formula • A chemical formula, like CO 2 or Na. Cl, indicates the relative number of atoms of each kind in a chemical compound. • For a MOLECULAR COMPOUND, the chemical formula gives the number of atoms of each element in a single molecule of that substance. C 8 H 18 Subscript indicates that there are 8 carbon atoms in a molecule of octane Subscript indicates that there are 18 hydrogen atoms in a molecule of octane

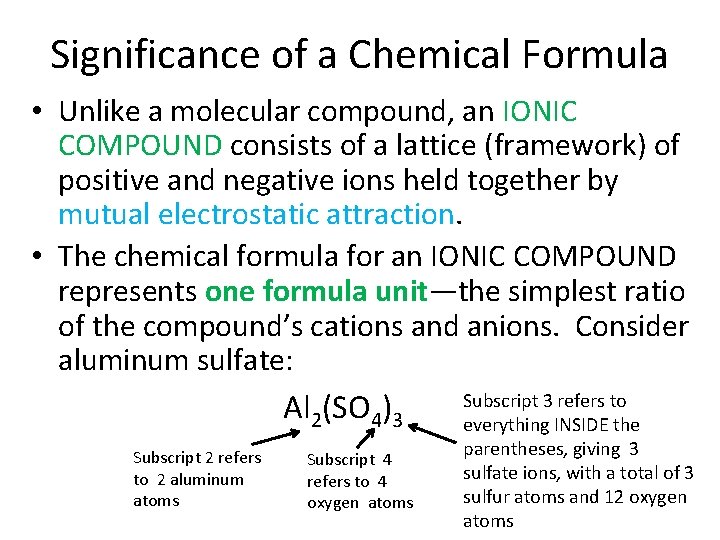
Significance of a Chemical Formula • Unlike a molecular compound, an IONIC COMPOUND consists of a lattice (framework) of positive and negative ions held together by mutual electrostatic attraction. • The chemical formula for an IONIC COMPOUND represents one formula unit—the simplest ratio of the compound’s cations and anions. Consider aluminum sulfate: Subscript 3 refers to Al 2(SO 4)3 everything INSIDE the Subscript 2 refers to 2 aluminum atoms Subscript 4 refers to 4 oxygen atoms parentheses, giving 3 sulfate ions, with a total of 3 sulfur atoms and 12 oxygen atoms

So how do I tell if a compound is IONIC or MOLECULAR? You will usually be able to tell by two simple rules: 1. If one element is a METAL and the other is a NONMETAL, it is usually an IONIC compound. 2. If both elements are NONMETALS, it is usually a MOLECULAR compound. There are exceptions (of course!): a) NH 4 Cl is an ionic compound, even though it is made of nonmetals. Remember that NH 4+ is a polyatomic cation. b) Compounds of beryllium are NEVER ionic because beryllium only shares electrons, so its compounds are always molecular.

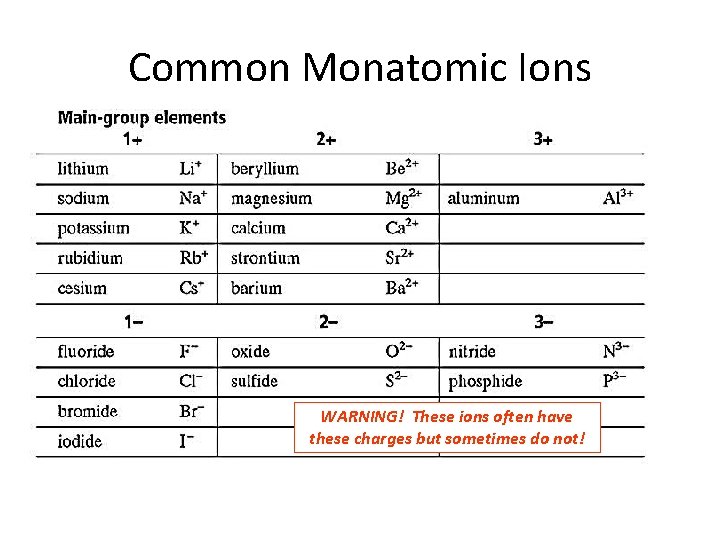
Monatomic Ions • Ions formed from a single atom are known as monatomic ions. • Many main group elements form ions with noblegas configurations by gaining or losing electrons. § Group 1 metals lose 1 e- to form 1+ cations § Group 2 metals lose 2 e- to form 2+ cations § Group 15 nonmetals gain 3 e- to form 3 - anions § Group 16 oxygen family gain 2 e- to form 2 - anions § Group 17 halogens gain 1 e- to form 1 - anions

Common Monatomic Ions WARNING! These ions often have these charges but sometimes do not!

Monatomic Ions • However, not all main group elements readily form ions. • Rather than gain or lose electrons, atoms of carbon and silicon form covalent bonds (share electrons). • Other elements form ions which do NOT have a noble-gas configuration. Tin (Sn) and lead (Pb) in Group 14 tend to lose their outermost p-orbital electrons but hang onto their s-orbital electrons and so form 2+ cations. However, they can also form covalent bonds in which their outer 4 electrons are shared, forming 4+ cations. These elements can form MULTIPLE ions. More on them later.

Naming Monatomic Ions • Monatomic cations are identified simply by the element’s name. K+ is the potassium cation. • Monatomic anions have a simple change: the ending of the element’s name is dropped from the root name and replaced by the suffix ‘-ide’. Fluorine (F) becomes the fluoride anion (F-) Nitrogen (N) becomes the nitride anion (N 3 -)

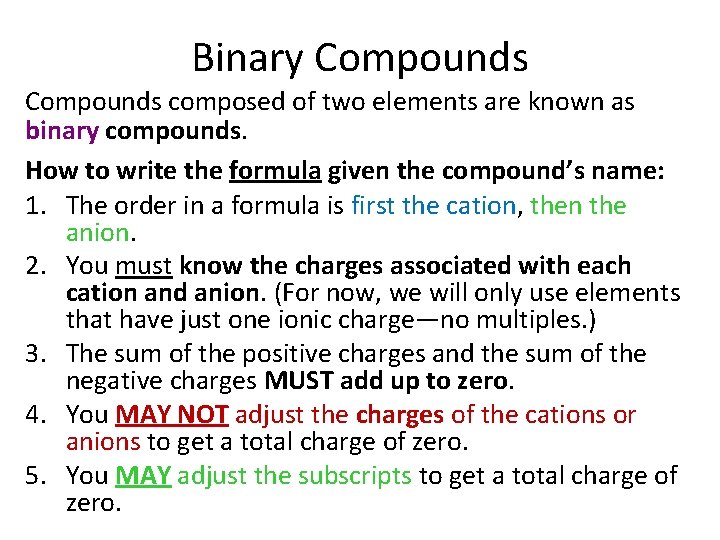
Binary Compounds composed of two elements are known as binary compounds. How to write the formula given the compound’s name: 1. The order in a formula is first the cation, then the anion. 2. You must know the charges associated with each cation and anion. (For now, we will only use elements that have just one ionic charge—no multiples. ) 3. The sum of the positive charges and the sum of the negative charges MUST add up to zero. 4. You MAY NOT adjust the charges of the cations or anions to get a total charge of zero. 5. You MAY adjust the subscripts to get a total charge of zero.

Example 1: Write the formula from the following name: sodium bromide 1. Write down the symbol and charge of the first element (cation). Result = Na+ 2. Write down the symbol and charge of the second element (anion). Result = Br¯ 3. Use the minimum number of cations and anions needed to make the sum of all charges in the formula equal zero. In this case, only one Na+ and one Br¯ are required. 4. The resulting formula is Na. Br.

Example 2: Write the formula from the following name: aluminum chloride 1. Write down the symbol and charge of the first element (cation). Result = Al 3+ 2. Write down the symbol and charge of the second element (anion). Result = Cl¯ 3. Use the minimum number of cations and anions needed to make the sum of all charges in the formula equal zero. In this case, only one Al 3+ is required, but three Cl¯ are required. Why? Three 1 - charges are required because there is one 3+ charge. Only in this way can the total charge of the formula be zero. 4. The resulting formula is Al. Cl 3.

Example 3: Write the formula of the following name: magnesium oxide 1. Write down the symbol and charge of the first element (cation). Result = Mg 2+ 2. Write down the symbol and charge of the second element (anion). Result = O 2¯ 3. Use the minimum number of cations and anions needed to make the sum of all charges in the formula equal zero. In this case, one Mg 2+ is required, as well as one O 2¯. Why? One 2+ charge is counterbalanced by one 2 charge. This gives a zero total charge for the formula. 4. The resulting formula is Mg. O.

Example 4: Write the formula of the following name: aluminum oxide 1. Write down the symbol and charge of the first element (cation). Result = Al 3+ 2. Write down the symbol and charge of the second element (anion). Result = O 2¯ 3. Use the minimum number of cations and anions needed to make the sum of all charges in the formula equal zero. Also, keep in mind that you cannot change the charges to make a formula correct.

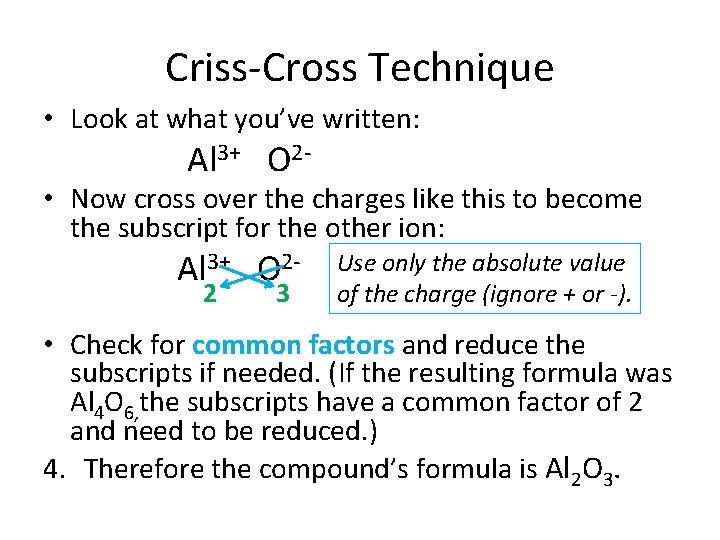
Criss-Cross Technique • Look at what you’ve written: Al 3+ O 2 - • Now cross over the charges like this to become the subscript for the other ion: Al 3+ O 22 3 Use only the absolute value of the charge (ignore + or -). • Check for common factors and reduce the subscripts if needed. (If the resulting formula was Al 4 O 6, the subscripts have a common factor of 2 and need to be reduced. ) 4. Therefore the compound’s formula is Al 2 O 3.

Binary Ionic Compounds How to write the name given the compound’s formula: 1. The order for names in a binary compound is first the cation, then the anion. 2. Use the name of the cation with a fixed oxidation state directly from the periodic table. 3. The name of the anion will be made from the root of the element's name plus the suffix " -ide. "

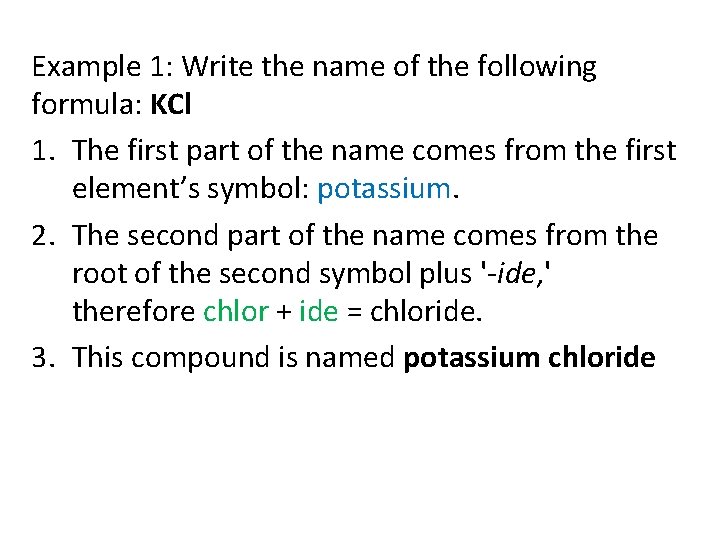
Example 1: Write the name of the following formula: KCl 1. The first part of the name comes from the first element’s symbol: potassium. 2. The second part of the name comes from the root of the second symbol plus '-ide, ' therefore chlor + ide = chloride. 3. This compound is named potassium chloride

Example 2: Write the name of the following formula: H 2 S 1. Look at first element and name it. Result of this step = hydrogen. 2. Look at second element. Use the root of its full name (which is sulf-) plus the ending "-ide. " Result of this step = sulfide. 3. These two steps give the full name of hydrogen sulfide. • Notice that the presence of the subscript is ignored. There are other types of binary compounds where you must pay attention to the subscript. Those are molecular compounds.

Example 3: Write the name of the following formula: Mg. Br 2 1. Look at first element and name it. Result of this step = magnesium. 2. Look at second element. Use root of its full name ( which is brom-) plus the ending "ide. " Result of this step = bromide. 3. This compound is named magnesium bromide. • Note the presence of the subscript does not play a role in this name.

• Worksheet on naming binary ionic compounds with fixed charges

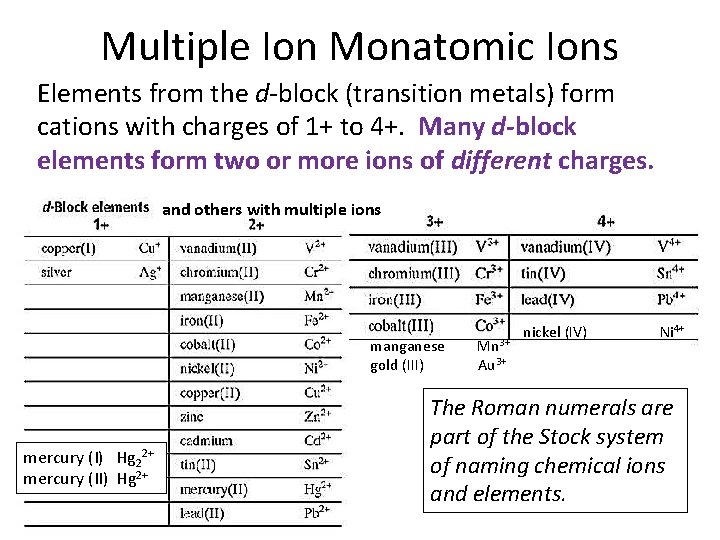
Multiple Ion Monatomic Ions Elements from the d-block (transition metals) form cations with charges of 1+ to 4+. Many d-block elements form two or more ions of different charges. and others with multiple ions manganese Mn 3+ gold (III) Au 3+ mercury (I) Hg 22+ mercury (II) Hg 2+ nickel (IV) Ni 4+ The Roman numerals are part of the Stock system of naming chemical ions and elements.

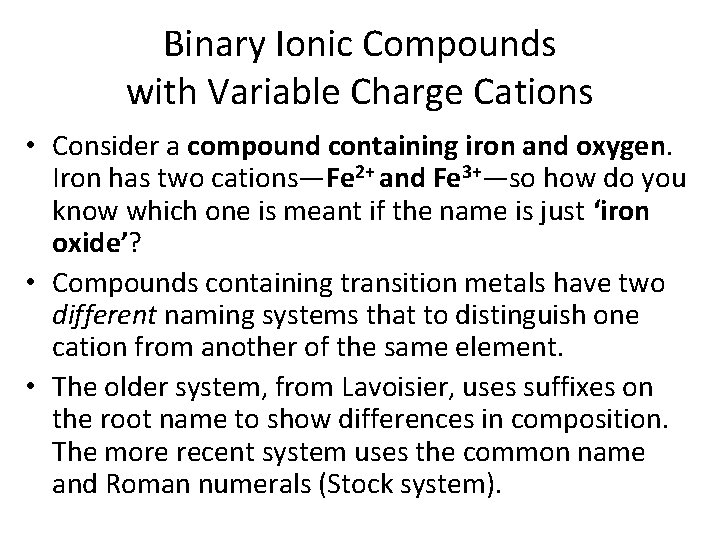
Binary Ionic Compounds with Variable Charge Cations • Consider a compound containing iron and oxygen. Iron has two cations—Fe 2+ and Fe 3+—so how do you know which one is meant if the name is just ‘iron oxide’? • Compounds containing transition metals have two different naming systems that to distinguish one cation from another of the same element. • The older system, from Lavoisier, uses suffixes on the root name to show differences in composition. The more recent system uses the common name and Roman numerals (Stock system).

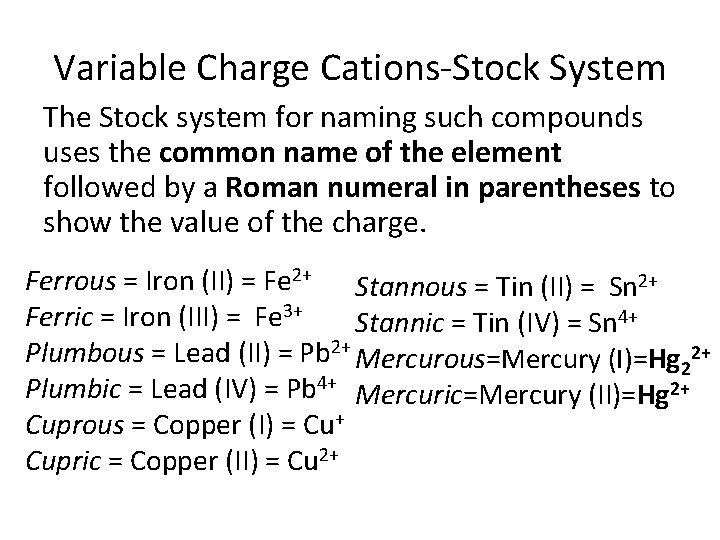
Variable Charge Cations-Stock System The Stock system for naming such compounds uses the common name of the element followed by a Roman numeral in parentheses to show the value of the charge. Ferrous = Iron (II) = Fe 2+ Stannous = Tin (II) = Sn 2+ Ferric = Iron (III) = Fe 3+ Stannic = Tin (IV) = Sn 4+ Plumbous = Lead (II) = Pb 2+ Mercurous=Mercury (I)=Hg 22+ Plumbic = Lead (IV) = Pb 4+ Mercuric=Mercury (II)=Hg 2+ Cuprous = Copper (I) = Cu+ Cupric = Copper (II) = Cu 2+

Example 1: Write the name of the following formula: Fe. O 1. “Fe” is iron. This will be iron (? ? ). 2. The anion here is oxide, O 2 -. 3. Is the cation Fe 2+ or Fe 3+ ? Look at the ions: Fe? O 2 - Which charge would balance? Correct, Fe 2+. The name is therefore iron (II) oxide.

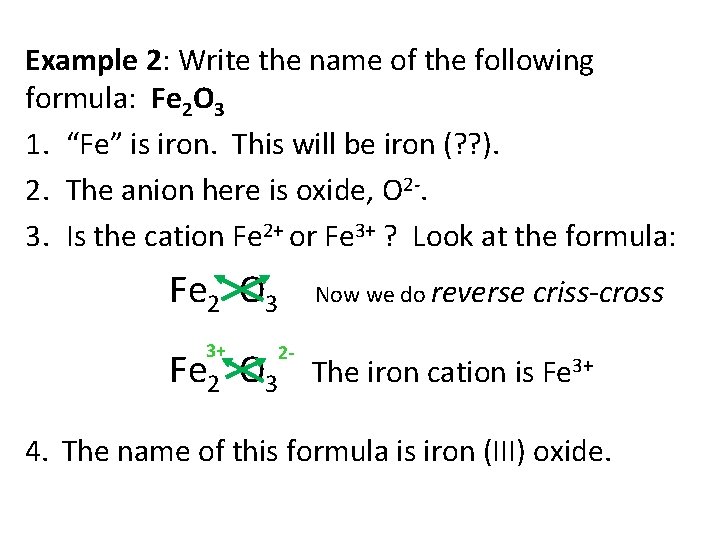
Example 2: Write the name of the following formula: Fe 2 O 3 1. “Fe” is iron. This will be iron (? ? ). 2. The anion here is oxide, O 2 -. 3. Is the cation Fe 2+ or Fe 3+ ? Look at the formula: Fe 2 O 3 Now we do reverse criss-cross 3+ 2 - Fe 2 O 3 The iron cation is Fe 3+ 4. The name of this formula is iron (III) oxide.

• Worksheet on naming binary compounds with variable cation charges

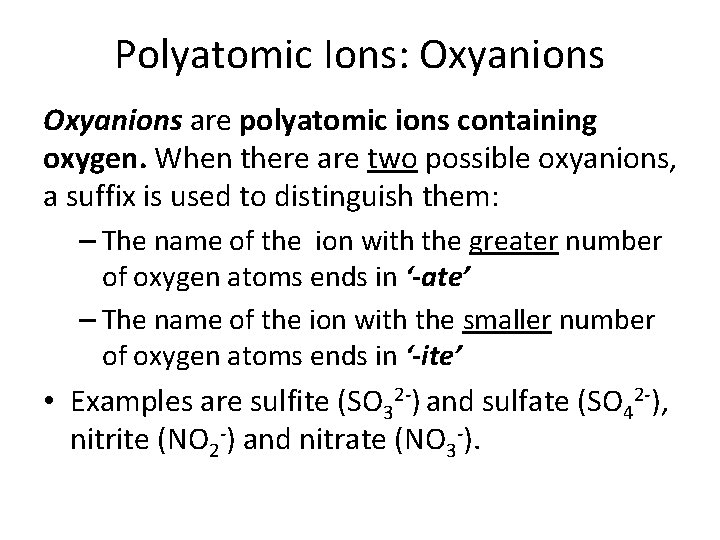
Polyatomic Ions: Oxyanions are polyatomic ions containing oxygen. When there are two possible oxyanions, a suffix is used to distinguish them: – The name of the ion with the greater number of oxygen atoms ends in ‘-ate’ – The name of the ion with the smaller number of oxygen atoms ends in ‘-ite’ • Examples are sulfite (SO 32 -) and sulfate (SO 42 -), nitrite (NO 2 -) and nitrate (NO 3 -).

Elements Forming More Than 2 Types of Oxyanions Cl. O- hypochlorite Cl. O 2 - chlorite Cl. O 3 - chlorate Cl. O 4 - perchlorate Chlorine and oxygen have 4 possible oxyanions. To keep them straight, we use two prefixes along with the ‘-ite’ and ‘-ate’ suffixes: ‘hypo-’ indicates one LESS oxygen than the chlorite ion has ‘per-’ indicates one MORE oxygen than the chlorate ion has

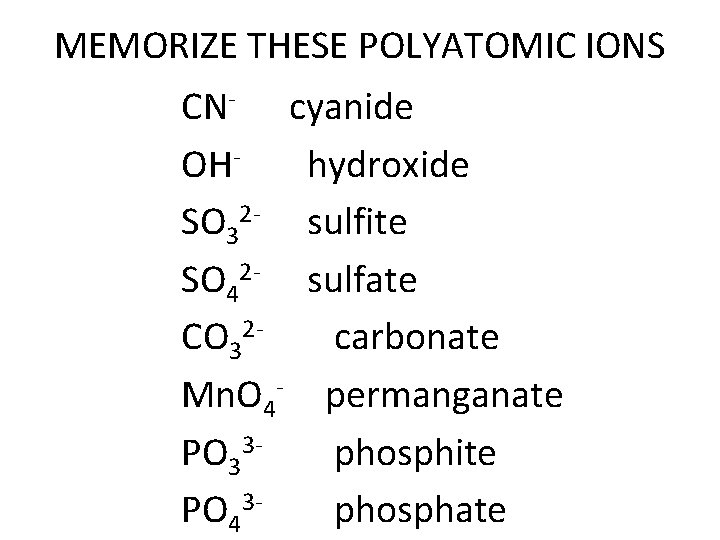
MEMORIZE THESE POLYATOMIC IONS CN- cyanide OH- hydroxide SO 32 - sulfite SO 42 - sulfate CO 32 carbonate Mn. O 4 - permanganate PO 33 phosphite PO 43 phosphate

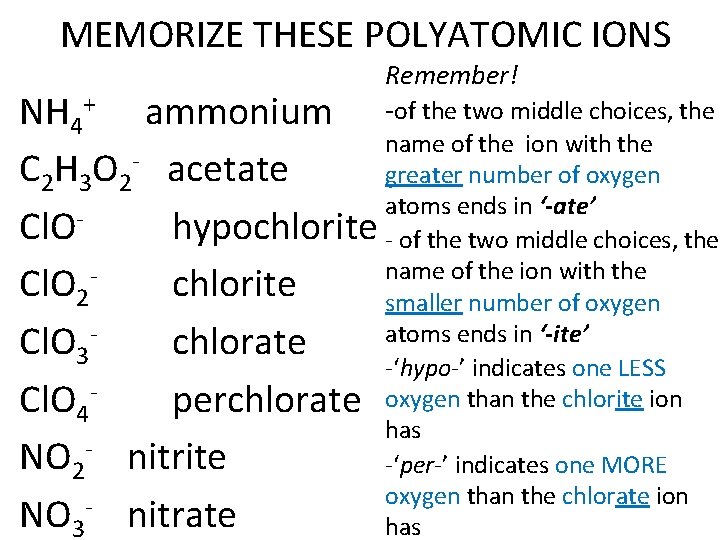
MEMORIZE THESE POLYATOMIC IONS Remember! NH 4+ ammonium -of the two middle choices, the name of the ion with the C 2 H 3 O 2 - acetate greater number of oxygen atoms ends in ‘-ate’ Cl. O hypochlorite - of the two middle choices, the name of the ion with the Cl. O 2 chlorite smaller number of oxygen atoms ends in ‘-ite’ Cl. O 3 chlorate -‘hypo-’ indicates one LESS Cl. O 4 perchlorate oxygen than the chlorite ion has NO 2 - nitrite -‘per-’ indicates one MORE oxygen than the chlorate ion NO 3 nitrate has

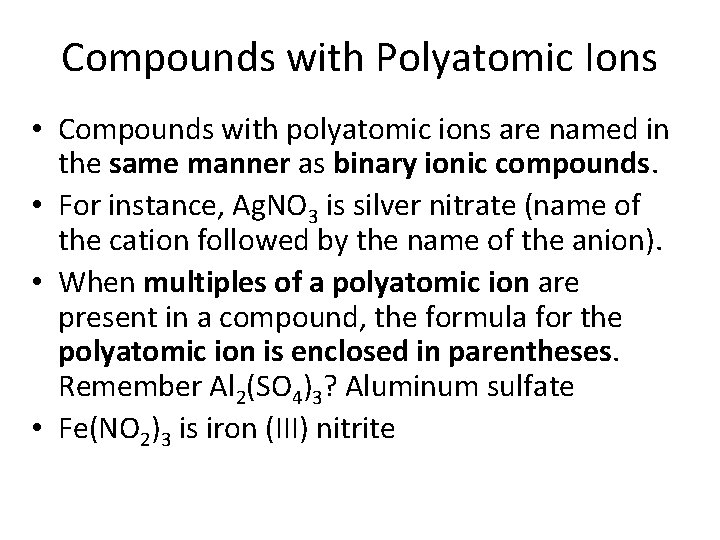
Compounds with Polyatomic Ions • Compounds with polyatomic ions are named in the same manner as binary ionic compounds. • For instance, Ag. NO 3 is silver nitrate (name of the cation followed by the name of the anion). • When multiples of a polyatomic ion are present in a compound, the formula for the polyatomic ion is enclosed in parentheses. Remember Al 2(SO 4)3? Aluminum sulfate • Fe(NO 2)3 is iron (III) nitrite

• Polyatomic ions WS half-sheet Conceptual chem Full sheet pre. AP chem

Binary Molecular Compounds • Remember, molecular compounds are composed of molecules, not formula units as in ionic compounds. • There are two systems of naming binary molecules, but we are going to learn just one now (the older system, based on the use of Greek prefixes). • For example, the molecular compound CCl 4 is named carbon tetrachloride. The prefix tetra- indicates that four chloride atoms are present in a single molecule of the compound.

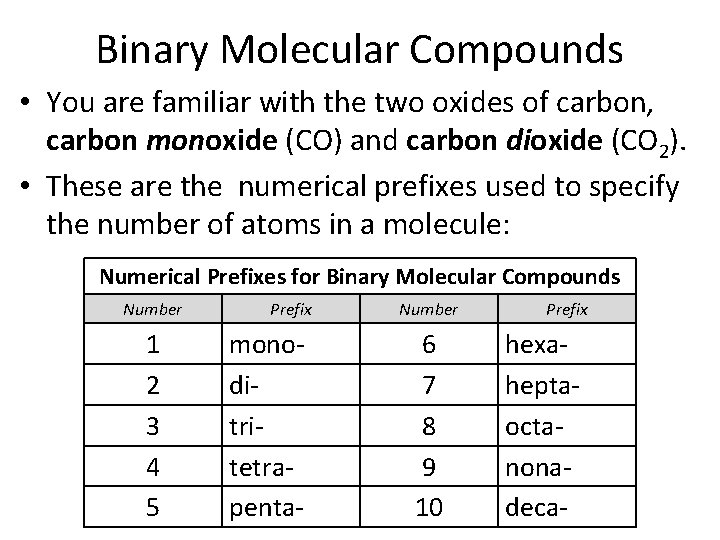
Binary Molecular Compounds • You are familiar with the two oxides of carbon, carbon monoxide (CO) and carbon dioxide (CO 2). • These are the numerical prefixes used to specify the number of atoms in a molecule: Numerical Prefixes for Binary Molecular Compounds Number 1 2 3 4 5 Prefix monoditritetrapenta- Number 6 7 8 9 10 Prefix hexaheptaoctanonadeca-

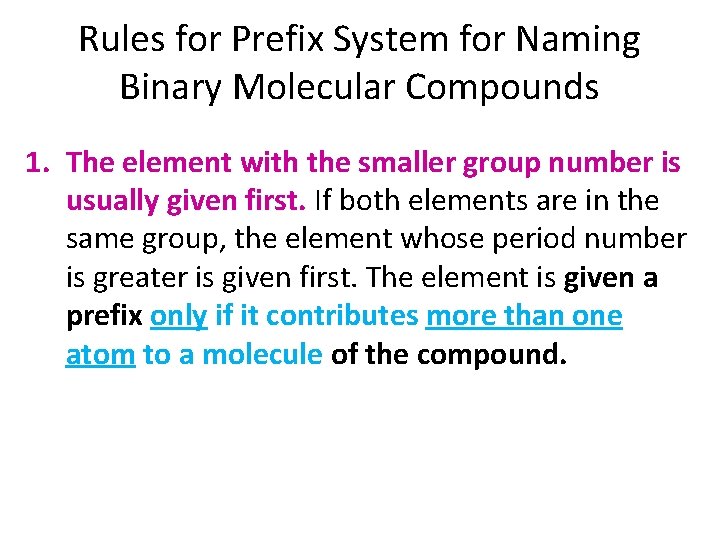
Rules for Prefix System for Naming Binary Molecular Compounds 1. The element with the smaller group number is usually given first. If both elements are in the same group, the element whose period number is greater is given first. The element is given a prefix only if it contributes more than one atom to a molecule of the compound.

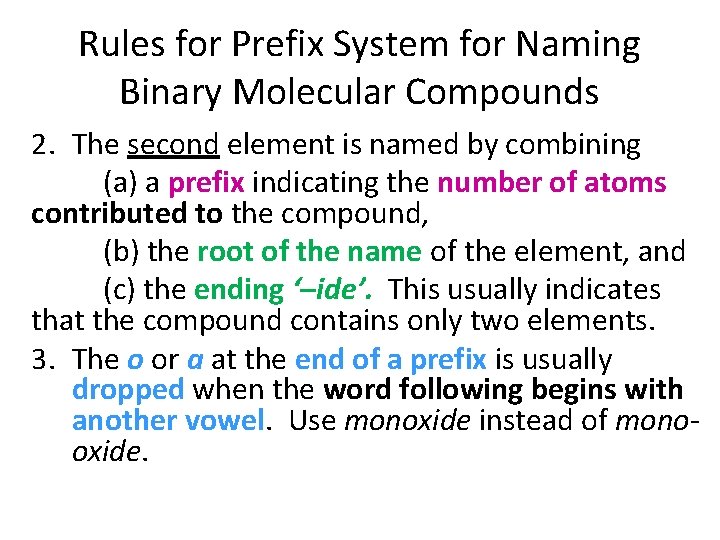
Rules for Prefix System for Naming Binary Molecular Compounds 2. The second element is named by combining (a) a prefix indicating the number of atoms contributed to the compound, (b) the root of the name of the element, and (c) the ending ‘–ide’. This usually indicates that the compound contains only two elements. 3. The o or a at the end of a prefix is usually dropped when the word following begins with another vowel. Use monoxide instead of monooxide.

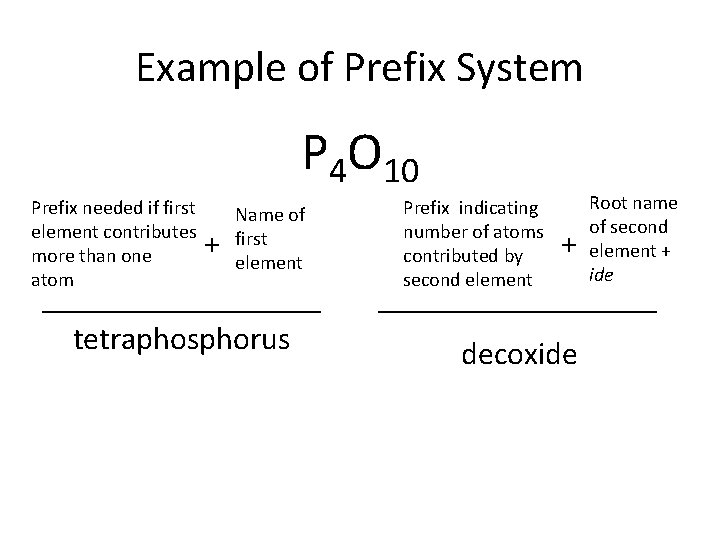
Example of Prefix System P 4 O 10 Prefix needed if first element contributes more than one atom + Name of first element tetraphosphorus Prefix indicating number of atoms contributed by second element + decoxide Root name of second element + ide

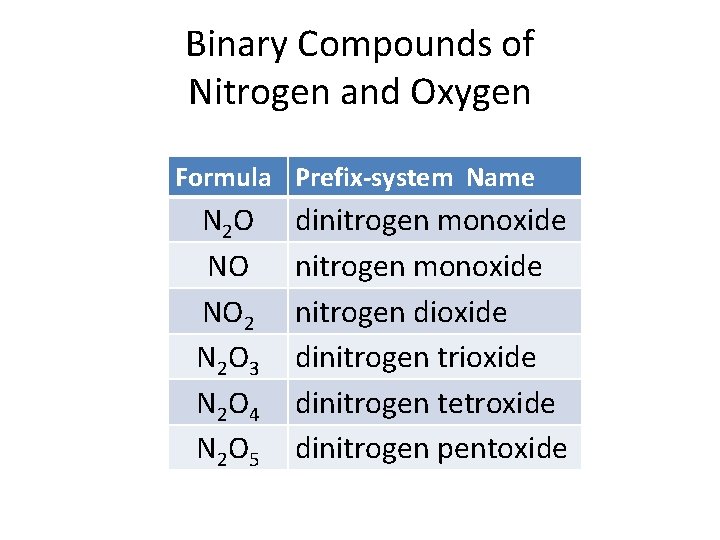
Binary Compounds of Nitrogen and Oxygen Formula Prefix-system Name N 2 O NO NO 2 N 2 O 3 N 2 O 4 N 2 O 5 dinitrogen monoxide nitrogen dioxide dinitrogen trioxide dinitrogen tetroxide dinitrogen pentoxide

As 2 O 5 More Examples a) More than one of the first element, so add prefix ‘di-’ to name of element = diarsenic b) More than one of the second element, so add prefix ‘pent-’ to name of element (element name starts with a vowel) = pentoxide As 2 O 5 is diarsenic pentoxide. Oxygen difluoride a) No prefix, so only one oxygen atom. b) Prefix ‘di-’ in difluoride shows there are two fluorine atoms. Oxygen difluoride has the formula OF 2.

• Worksheet on binary molecular compounds

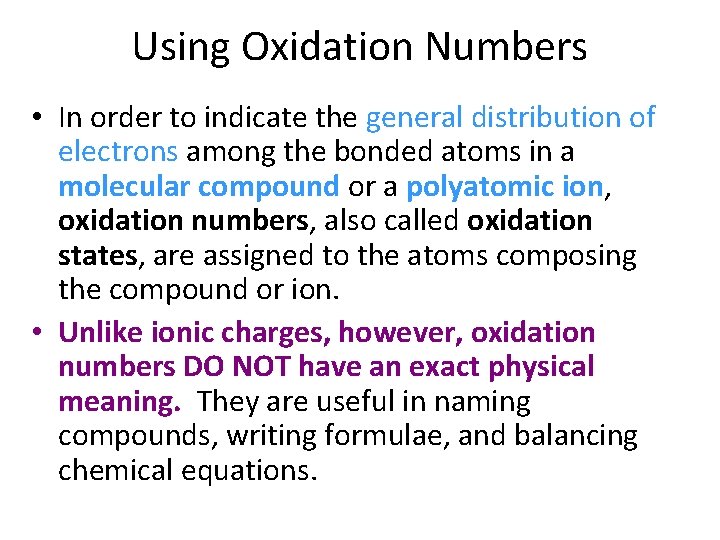
Using Oxidation Numbers • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, oxidation numbers, also called oxidation states, are assigned to the atoms composing the compound or ion. • Unlike ionic charges, however, oxidation numbers DO NOT have an exact physical meaning. They are useful in naming compounds, writing formulae, and balancing chemical equations.

Rules for Assigning Oxidation #s to Covalently Bonded Ions 1. In general, shared electrons are assumed to belong to the more electronegative atom in each bond. 2. The atoms in a pure element have an oxidation number of zero. (True for Na, O 2) 3. The more-electronegative element in a binary molecular compound is assigned the number equal to the negative charge it would have as an anion.

Rules for Assigning Oxidation #s to Covalently Bonded Ions 4. The less-electronegative element is assigned the number equal to the positive charge it would have as a cation. 5. Fluorine has an oxidation number of -1 in ALL of its compounds because it is the most electronegative element. 6. Oxygen has an oxidation number of -2 in ALMOST all of its compounds. Exceptions occur when it is in peroxides such as H 2 O 2 (then -1) and in compounds with fluorine such as OF 2 (then +2).

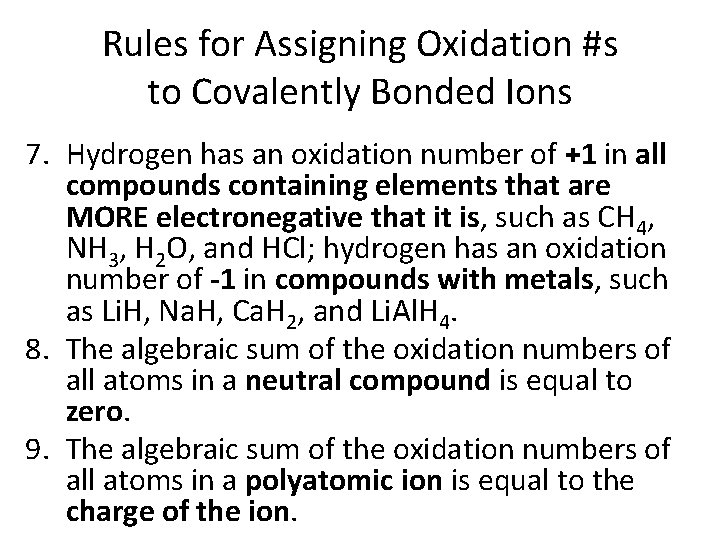
Rules for Assigning Oxidation #s to Covalently Bonded Ions 7. Hydrogen has an oxidation number of +1 in all compounds containing elements that are MORE electronegative that it is, such as CH 4, NH 3, H 2 O, and HCl; hydrogen has an oxidation number of -1 in compounds with metals, such as Li. H, Na. H, Ca. H 2, and Li. Al. H 4. 8. The algebraic sum of the oxidation numbers of all atoms in a neutral compound is equal to zero. 9. The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.

Can You Use Oxidation Numbers in Ionic Compounds? • Yes. A monatomic ion has an oxidation number equal to the charge of the ion. Na+ has an oxidation number of +1 Ca 2+ has an oxidation number of +2 Cl- has an oxidation number of -1

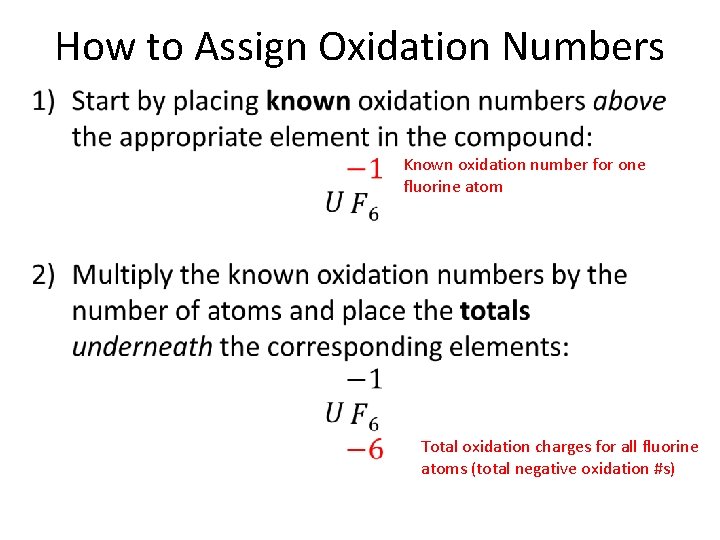
How to Assign Oxidation Numbers • Known oxidation number for one fluorine atom Total oxidation charges for all fluorine atoms (total negative oxidation #s)

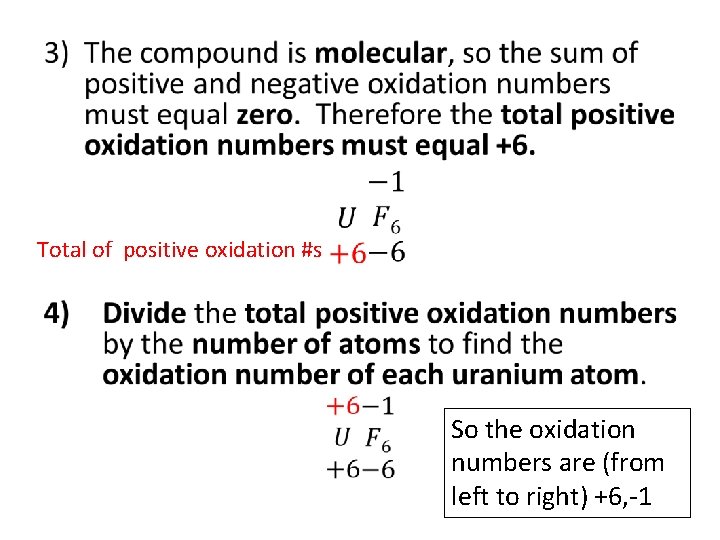
• Total of positive oxidation #s So the oxidation numbers are (from left to right) +6, -1

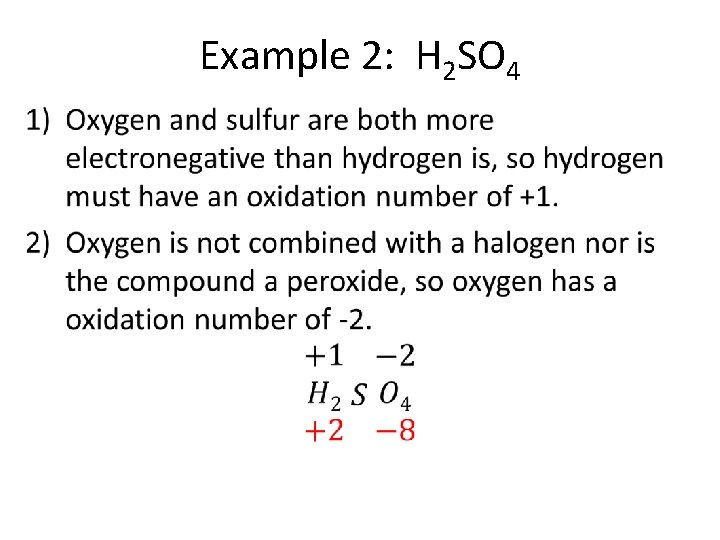
Example 2: H 2 SO 4 •

• For the total positive oxidation numbers to equal +8, sulfur must have an oxidation number of +6

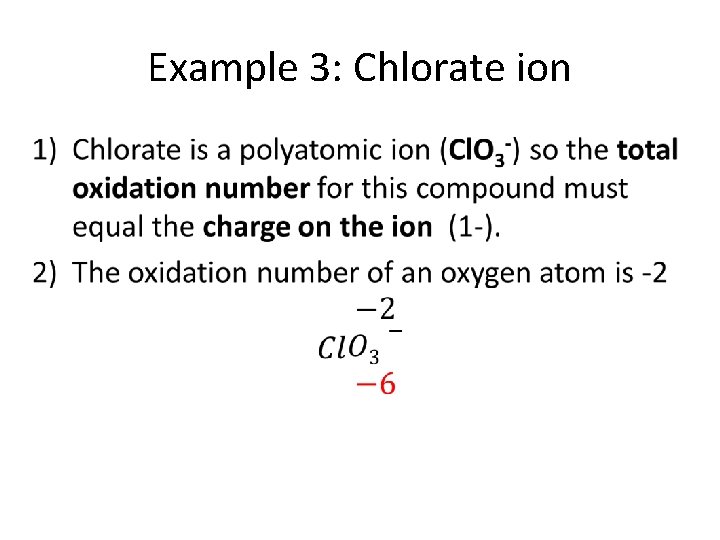
Example 3: Chlorate ion •

• Oxidation number of one chlorine atom Total must equal -1
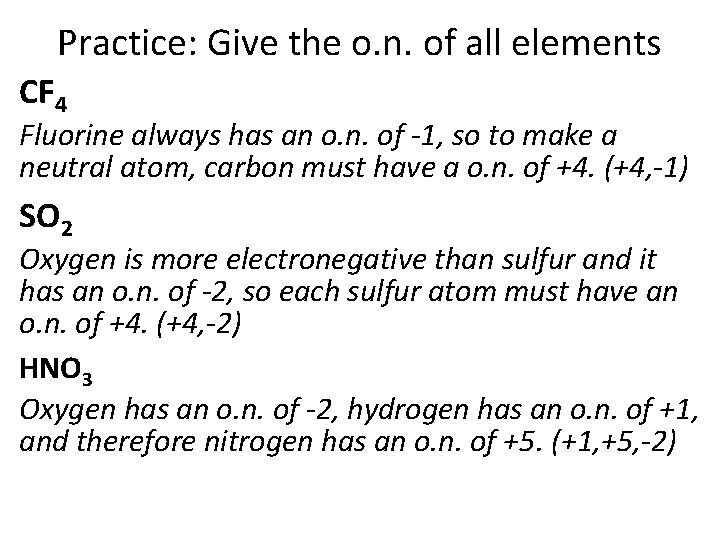
Practice: Give the o. n. of all elements CF 4 Fluorine always has an o. n. of -1, so to make a neutral atom, carbon must have a o. n. of +4. (+4, -1) SO 2 Oxygen is more electronegative than sulfur and it has an o. n. of -2, so each sulfur atom must have an o. n. of +4. (+4, -2) HNO 3 Oxygen has an o. n. of -2, hydrogen has an o. n. of +1, and therefore nitrogen has an o. n. of +5. (+1, +5, -2)

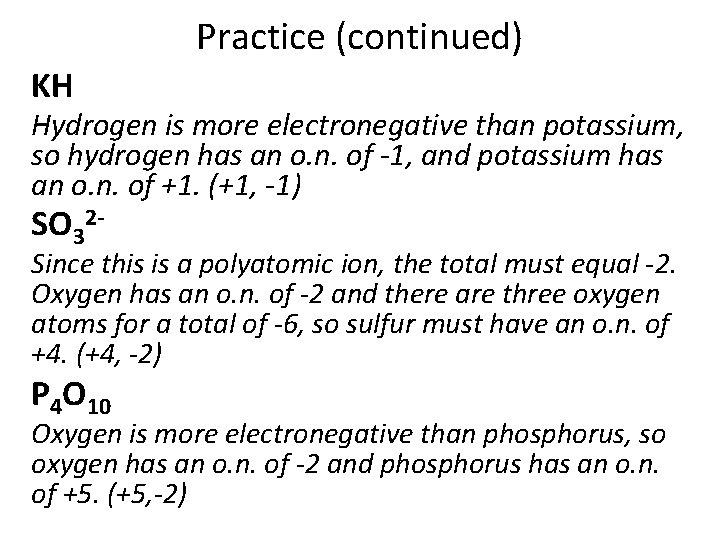
Practice (continued) KH Hydrogen is more electronegative than potassium, so hydrogen has an o. n. of -1, and potassium has an o. n. of +1. (+1, -1) SO 32 - Since this is a polyatomic ion, the total must equal -2. Oxygen has an o. n. of -2 and there are three oxygen atoms for a total of -6, so sulfur must have an o. n. of +4. (+4, -2) P 4 O 10 Oxygen is more electronegative than phosphorus, so oxygen has an o. n. of -2 and phosphorus has an o. n. of +5. (+5, -2)