Nomenclature of Organic Compounds Dr V Jeevana Jyothi




















- Slides: 56

Nomenclature of Organic Compounds Dr. V. Jeevana Jyothi Associate Professor Department of Chemistry RBVRR Women’s College

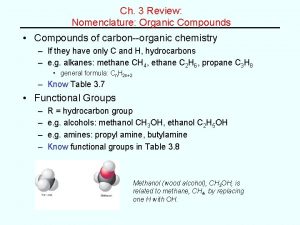
Organic Chemistry �It is the study of carbon compounds. �It is studied as a separate discipline because of the following reasons � 1. large number of compounds � 2. Unique Physical & chemical properties � 3. Unique character of Carbon-Catenation- carbon has the ability to bond successively to other carbon atoms to form chains.

Importance of Organic Chemistry in Everyday life � Food: Proteins, Fats, Carbohydrates � Clothing: Cotton, Silk, Wool, Nylon, rayon � Shelter: wood, paints, Varnishes � Power & Transportation: Natural gas, petroleum products, Coal � Medicines & Drugs: Penicillin, Streptomycin, Aspirin � Insecticides: DDT � Herbicides: Treflan � Hormones & Steroids � Vitamins & Enzymes � Antiseptics � Pigments & Dyes � Paper & Inks � Photographic films & Developers � Perfumes & Flavours � Plastics, rubber & resin � Propellants & Explosives � Soaps & Detergents � Refrigerants

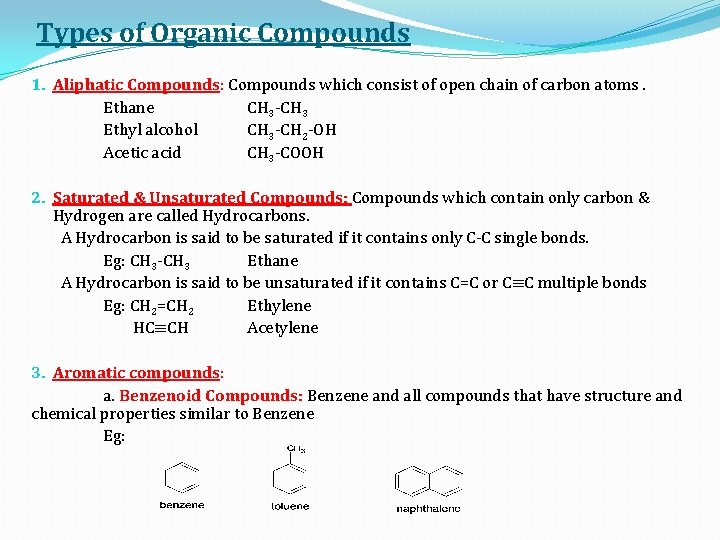
Types of Organic Compounds 1. Aliphatic Compounds: Compounds which consist of open chain of carbon atoms. Ethane CH 3 -CH 3 Ethyl alcohol CH 3 -CH 2 -OH Acetic acid CH 3 -COOH 2. Saturated & Unsaturated Compounds: Compounds which contain only carbon & Hydrogen are called Hydrocarbons. A Hydrocarbon is said to be saturated if it contains only C-C single bonds. Eg: CH 3 -CH 3 Ethane A Hydrocarbon is said to be unsaturated if it contains C=C or C≡C multiple bonds Eg: CH 2=CH 2 Ethylene HC≡CH Acetylene 3. Aromatic compounds: a. Benzenoid Compounds: Benzene and all compounds that have structure and chemical properties similar to Benzene Eg:

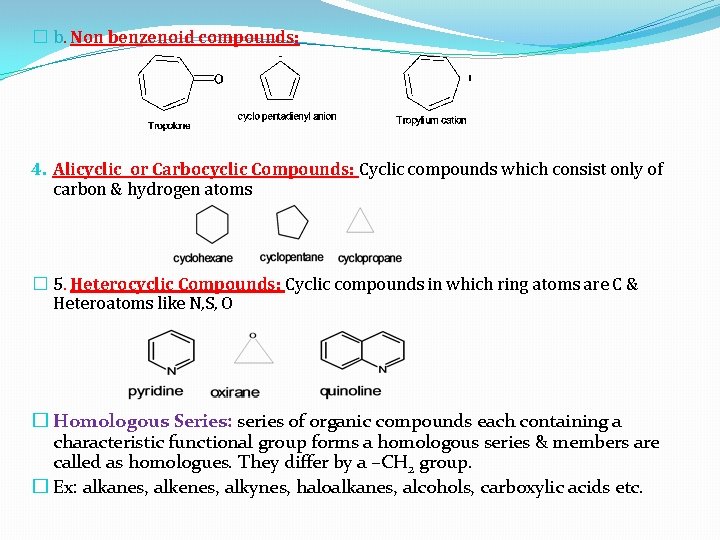
� b. Non benzenoid compounds: 4. Alicyclic or Carbocyclic Compounds: Cyclic compounds which consist only of carbon & hydrogen atoms � 5. Heterocyclic Compounds: Cyclic compounds in which ring atoms are C & Heteroatoms like N, S, O � Homologous Series: series of organic compounds each containing a characteristic functional group forms a homologous series & members are called as homologues. They differ by a –CH 2 group. � Ex: alkanes, alkenes, alkynes, haloalkanes, alcohols, carboxylic acids etc.

Representations of Organic Molecules n-Pentane: C 5 H 12 �Long Hand formula: CH 3 -CH 2 -CH 3 �Short hand formula: �Skeletal formula: C-C-C �Condensed Formula: CH 3 -(CH 2)3 -CH 3

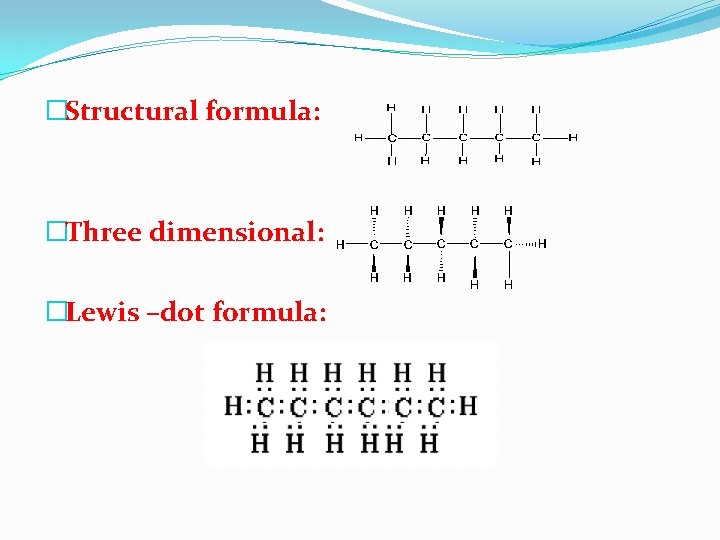
�Structural formula: �Three dimensional: �Lewis –dot formula:

Principles of Nomenclature � In early days , each new compound was given an individual name. Such name was based on the source, some property or some other trivial reason. � Eg: Formic acid, it was named as it was obtained by distillation of red ants. � Methane from marsh gas � Citric acid from citrus foods � Nitrobenzene, it is named as oil of mirbane � Curcumin from turmeric & many more…… � An ordinary name given to a compound without reference to its structure is called a common name or trivial name


�IUPAC System of Nomenclature: �With the rapid growth of organic chemistry, over 6 million of compounds were discovered. It was impossible to give common names. �In 1957, the International Union of Pure and Applied Chemistry proposed rules to give a systematic naming to organic compounds based on their structure. Each organic compound has only single IUPAC name. �According to IUPAC, name of an organic compound has the following parts � 1. Prefix: Indicates the identity, location, and number of substituents attached to the carbon chain � eg: Chloro, alkyl, amino, hydroxy etc. � 2. Root word : Represents the no. of carbon atoms in the continuous longest chain � 3. Primary suffix: Indicates the presence or absence of saturation � 4. Secondary Suffix: Indicates the functional group present

Root Word: � 1 -Meth � 2 -Eth � 3 -Prop � 4 -But � 5 -Pent � 6 -Hex � 7 -Hept � 8 -Oct � 9 -Non � 10 -Dec � 20 -Eicos Primary Suffix: Saturated with all single bonds: -ane Unsaturated with = bond: -ene Unsaturated with ≡ bond: -yne Secondary Suffix: indicates the functional group present

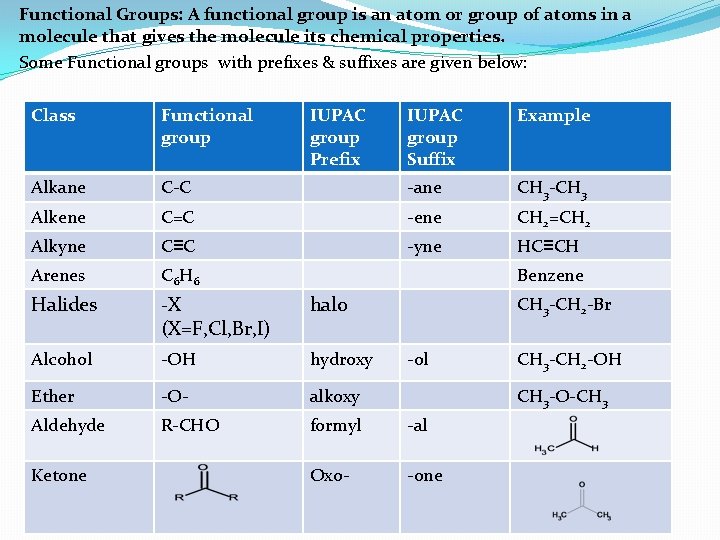
Functional Groups: A functional group is an atom or group of atoms in a molecule that gives the molecule its chemical properties. Some Functional groups with prefixes & suffixes are given below: Class Functional group Alkane IUPAC group Suffix Example C-C -ane CH 3 -CH 3 Alkene C=C -ene CH 2=CH 2 Alkyne C≡C -yne HC≡CH Arenes C 6 H 6 Halides -X (X=F, Cl, Br, I) halo Alcohol -OH hydroxy Ether -O- alkoxy Aldehyde R-CHO formyl -al Oxo- -one Ketone IUPAC group Prefix Benzene CH 3 -CH 2 -Br -ol CH 3 -CH 2 -OH CH 3 -O-CH 3

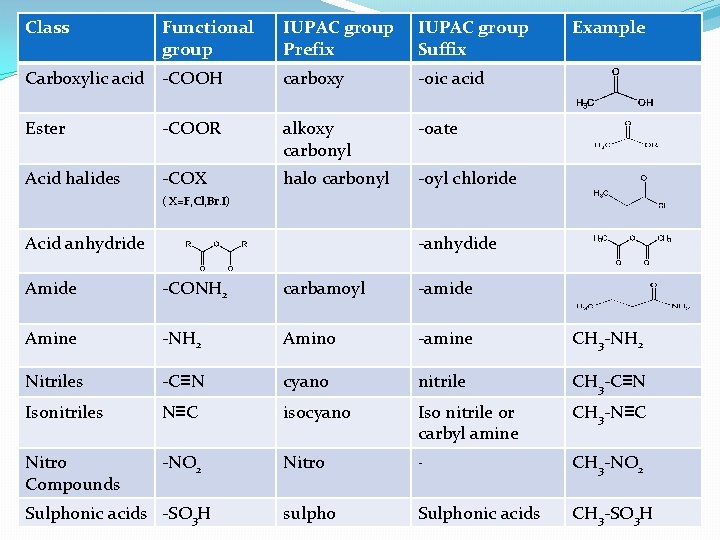
Class Functional group IUPAC group Prefix IUPAC group Suffix Carboxylic acid -COOH carboxy -oic acid Ester -COOR alkoxy carbonyl -oate Acid halides -COX halo carbonyl -oyl chloride Example ( X=F, Cl, Br. I) Acid anhydride -anhydide Amide -CONH 2 carbamoyl -amide Amine -NH 2 Amino -amine CH 3 -NH 2 Nitriles -C≡N cyano nitrile CH 3 -C≡N Isonitriles N≡C isocyano Iso nitrile or carbyl amine CH 3 -N≡C Nitro Compounds -NO 2 Nitro - CH 3 -NO 2 sulpho Sulphonic acids CH 3 -SO 3 H Sulphonic acids -SO 3 H

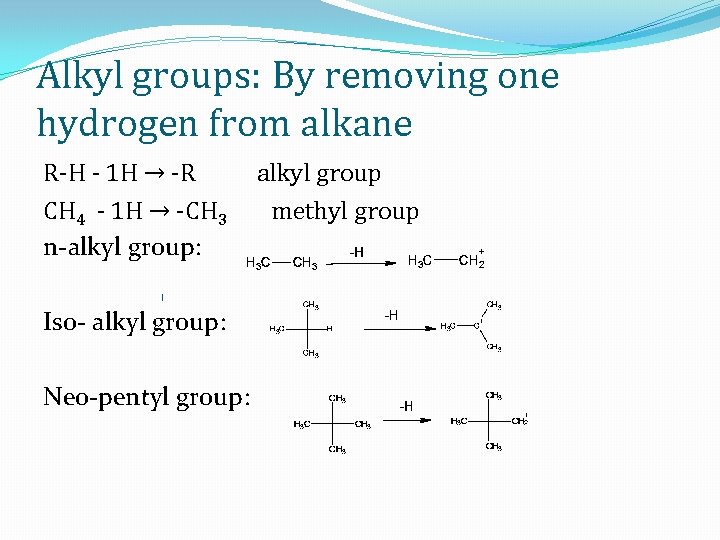
Alkyl groups: By removing one hydrogen from alkane R-H - 1 H → -R CH 4 - 1 H → -CH 3 n-alkyl group: Iso- alkyl group: Neo-pentyl group: alkyl group methyl group

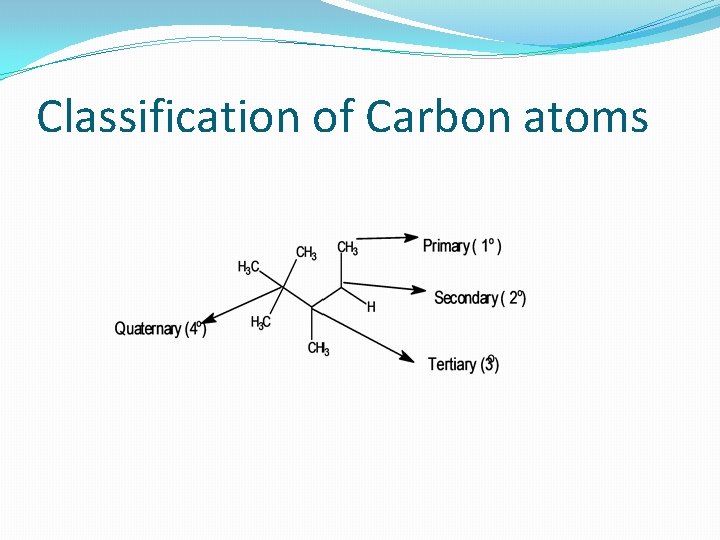
Classification of Carbon atoms

Alkyl group CH 3 - Structure CH 3 - IUPAC name ( Prefix) Abbreviation methyl Me- CH 3 CH 2 - ethyl Et- CH 3 CH 2 - n-propyl n-Pr CH 3 CHCH 3 isopropyl or i-propyl i-Pr CH 3 CH 2 CH 2 - n-butyl n-Bu CH 3 CH 2 CHCH 3 sec-butyl s-Bu (CH 3)2 CHCH 2 - isobutyl or i-butyl i-Bu (CH 3)3 C- tert-butyl or t-butyl t-Bu C 6 H 5 - phenyl Ph

ALKANES Names of first 10 straight chain alkanes Carbon atoms Name Molecular formula 1 Methane CH 4 2 Ethane C 2 H 6 3 Propane C 3 H 8 4 butane C 4 H 10 5 Penatne C 5 H 12 6 Hexane C 6 H 14 7 Heptane C 7 H 16 8 Octane C 8 H 18 9 Nonane C 9 H 20 10 Decane C 10 H 22

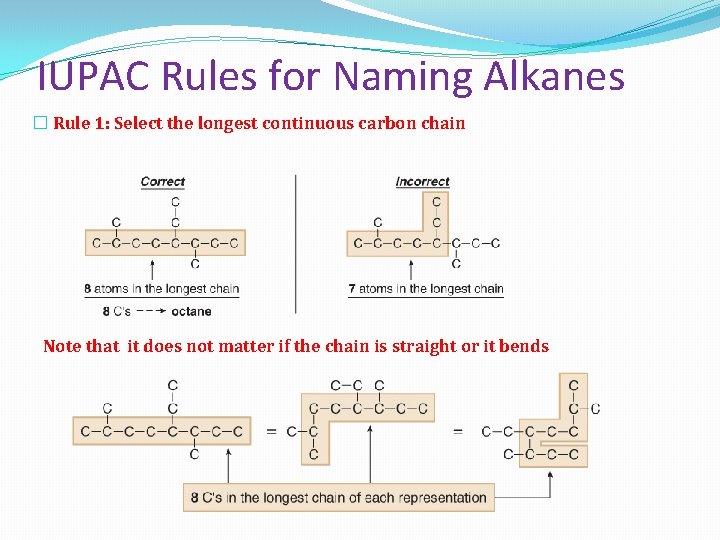
IUPAC Rules for Naming Alkanes � Rule 1: Select the longest continuous carbon chain Note that it does not matter if the chain is straight or it bends

Also note that if there are two chains of equal length, pick the chain with more substituents. In the following example, two different chains in the same alkane have seven C atoms. We circle the longest continuous chain as shown in the diagram on the left, since this results in the greater number of substituents.

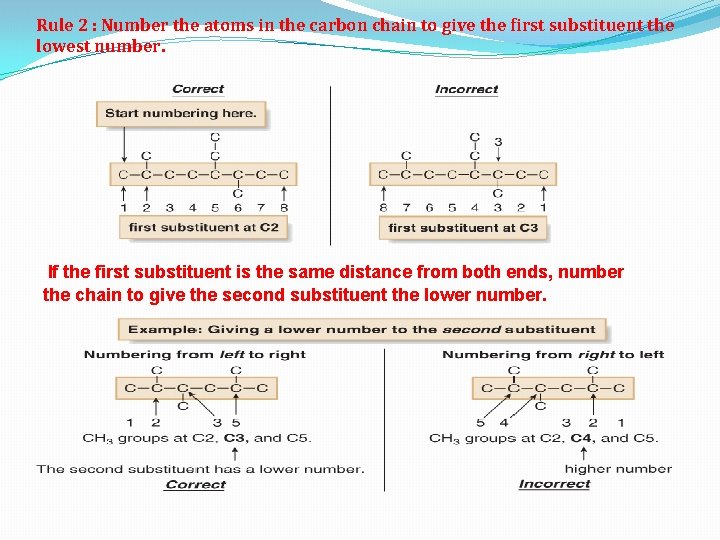
Rule 2 : Number the atoms in the carbon chain to give the first substituent the lowest number. If the first substituent is the same distance from both ends, number the chain to give the second substituent the lower number.

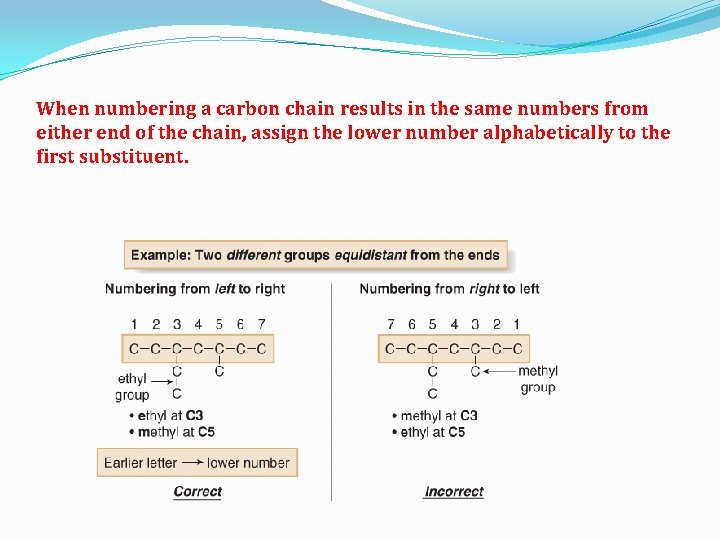
When numbering a carbon chain results in the same numbers from either end of the chain, assign the lower number alphabetically to the first substituent.

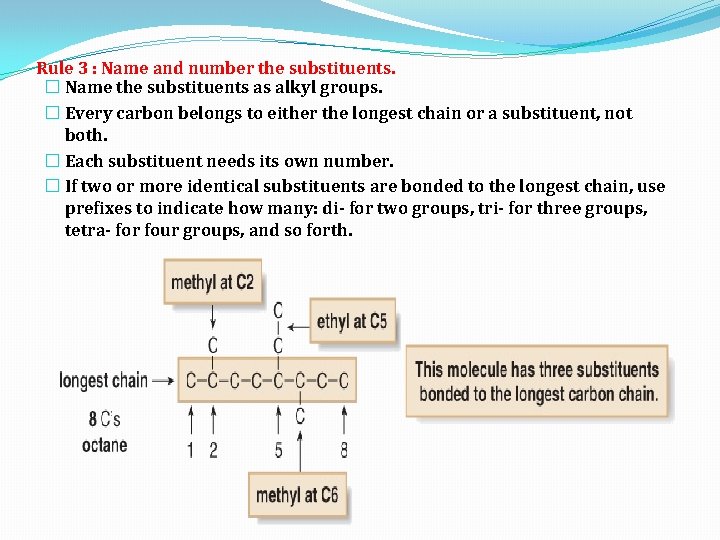
Rule 3 : Name and number the substituents. � Name the substituents as alkyl groups. � Every carbon belongs to either the longest chain or a substituent, not both. � Each substituent needs its own number. � If two or more identical substituents are bonded to the longest chain, use prefixes to indicate how many: di- for two groups, tri- for three groups, tetra- for four groups, and so forth.

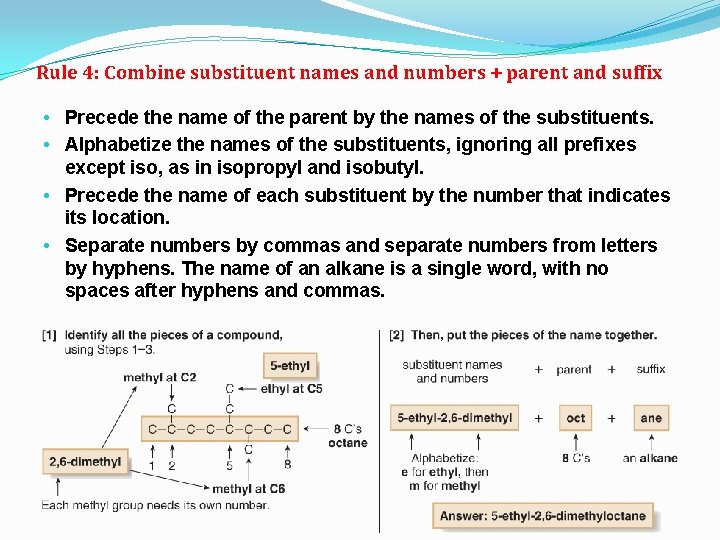
Rule 4: Combine substituent names and numbers + parent and suffix • Precede the name of the parent by the names of the substituents. • Alphabetize the names of the substituents, ignoring all prefixes except iso, as in isopropyl and isobutyl. • Precede the name of each substituent by the number that indicates its location. • Separate numbers by commas and separate numbers from letters by hyphens. The name of an alkane is a single word, with no spaces after hyphens and commas.

CYCLO ALKANES 1. Cycloalkanes are named by using similar rules, but the prefix cyclo- immediately precedes the name of the parent. Example:

2. Name and number the substituents. No number is needed to indicate the location of a single substituent. 3. For rings with more than one substituent, begin numbering at one substituent and proceed around the ring to give the second substituent the lowest number.

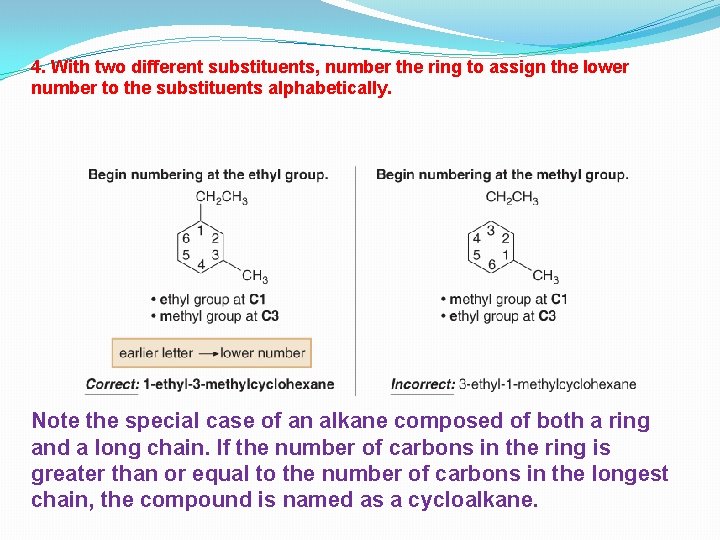
4. With two different substituents, number the ring to assign the lower number to the substituents alphabetically. Note the special case of an alkane composed of both a ring and a long chain. If the number of carbons in the ring is greater than or equal to the number of carbons in the longest chain, the compound is named as a cycloalkane.

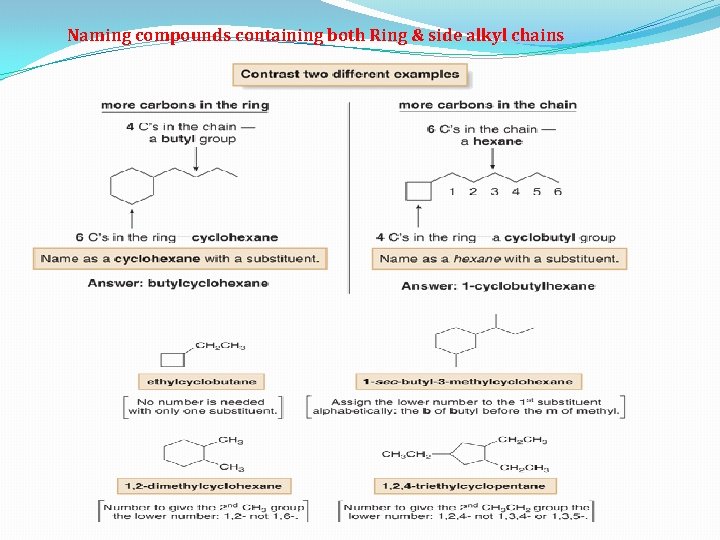
Naming compounds containing both Ring & side alkyl chains

ALKENES 1. Select the longest carbon chain containing the double bond 2. Name the longest chain- the name is obtained by dropping the final-ane from the name of corresponding alkane, & adding the ending –ene 3. Number the chain from the end closer to the double bond 4. Indicate the position of the double bond by the number of the first (lowest numbered) carbon atom involved in the db. 5. Alkyl groups and other substituents are numbered, named and placed as prefixes in the alphabetical order. 6. Alkenes containing two double bonds are named as alkadienes

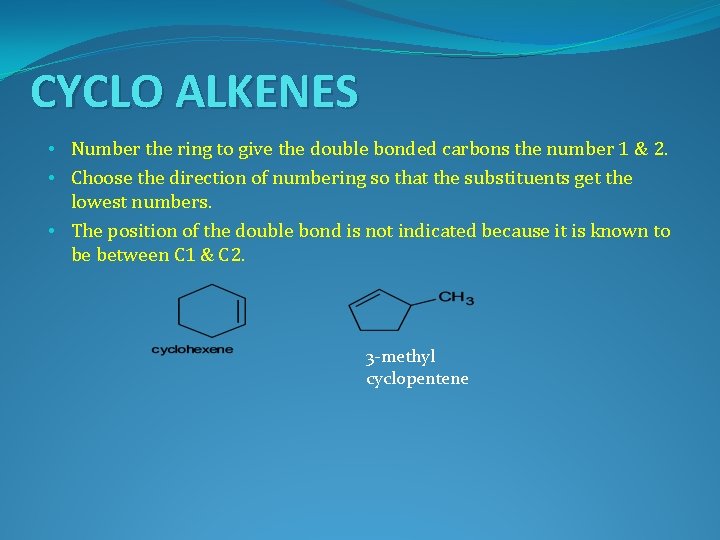
CYCLO ALKENES • Number the ring to give the double bonded carbons the number 1 & 2. • Choose the direction of numbering so that the substituents get the lowest numbers. • The position of the double bond is not indicated because it is known to be between C 1 & C 2. 3 -methyl cyclopentene

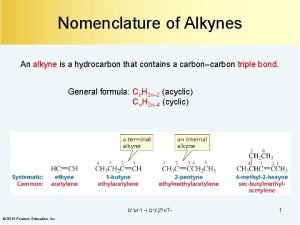
ALKYNES 1. Select the longest carbon chain containing the triple bond 2. Name the longest chain- the name is obtained by dropping the finalane from the name of corresponding alkane, & adding the ending – yne 3. Number the chain from the end closer to the triple bond 4. Indicate the position of the triple bond by the number of the first (lowest numbered) carbon atom involved in the db. 5. Alkyl groups and other substituents are numbered, named and placed as prefixes in the alphabetical order. 6. Alkynes containing two triple bonds are named as alkadiynes

Alkyl Halides 1. Select the longest chain to which the halogen is attached and give it the name of the corresponding alkane 2. Prefix the name of the alkane by chloro, bromo, iodo or fluoro 3. Number the chain so as to give the carbon carrying the halogen atom the lowest possible number 4. Other substituents are numbered, named and placed as prefixes in alphabetic order. If there are two or more identical halogen substituents, the prefixes di, tri, tetra , etc. . are used 2 -bromopropane

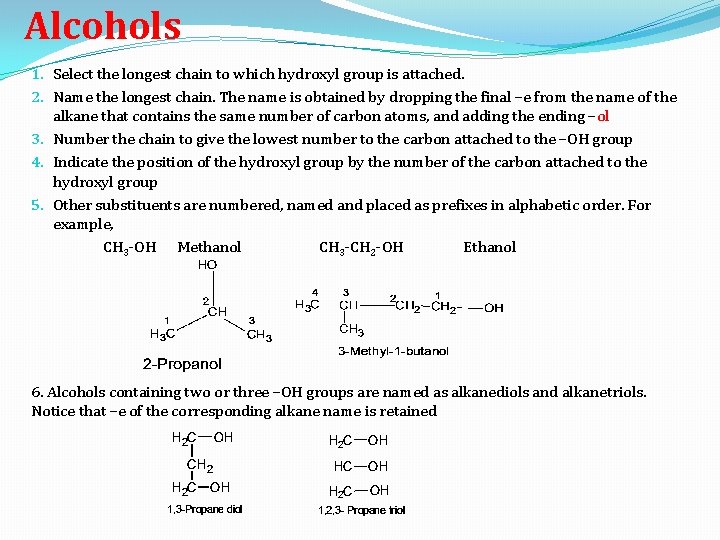
Alcohols 1. Select the longest chain to which hydroxyl group is attached. 2. Name the longest chain. The name is obtained by dropping the final –e from the name of the alkane that contains the same number of carbon atoms, and adding the ending –ol 3. Number the chain to give the lowest number to the carbon attached to the –OH group 4. Indicate the position of the hydroxyl group by the number of the carbon attached to the hydroxyl group 5. Other substituents are numbered, named and placed as prefixes in alphabetic order. For example, CH 3 -OH Methanol CH 3 -CH 2 -OH Ethanol 6. Alcohols containing two or three –OH groups are named as alkanediols and alkanetriols. Notice that –e of the corresponding alkane name is retained

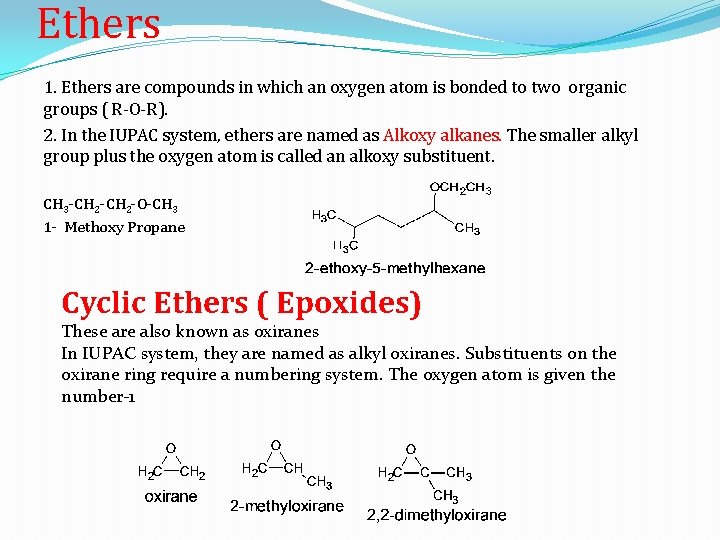
Ethers 1. Ethers are compounds in which an oxygen atom is bonded to two organic groups ( R-O-R). 2. In the IUPAC system, ethers are named as Alkoxy alkanes. The smaller alkyl group plus the oxygen atom is called an alkoxy substituent. CH 3 -CH 2 -O-CH 3 1 - Methoxy Propane Cyclic Ethers ( Epoxides) These are also known as oxiranes In IUPAC system, they are named as alkyl oxiranes. Substituents on the oxirane ring require a numbering system. The oxygen atom is given the number-1

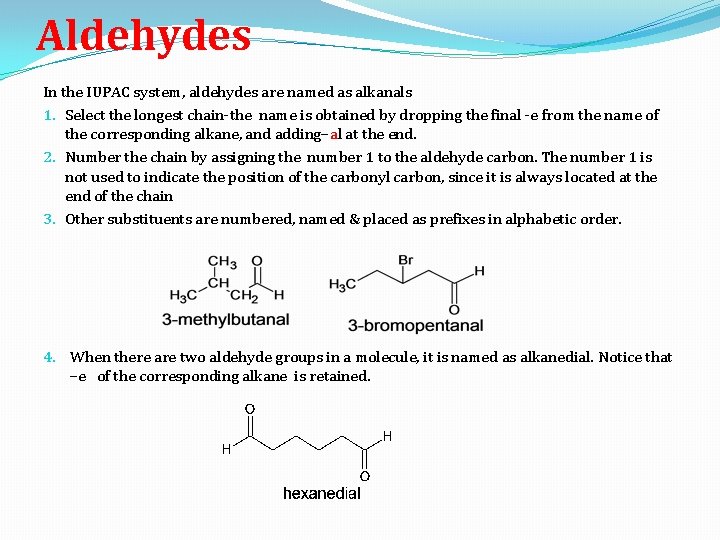
Aldehydes In the IUPAC system, aldehydes are named as alkanals 1. Select the longest chain-the name is obtained by dropping the final -e from the name of the corresponding alkane, and adding–al at the end. 2. Number the chain by assigning the number 1 to the aldehyde carbon. The number 1 is not used to indicate the position of the carbonyl carbon, since it is always located at the end of the chain 3. Other substituents are numbered, named & placed as prefixes in alphabetic order. 4. When there are two aldehyde groups in a molecule, it is named as alkanedial. Notice that –e of the corresponding alkane is retained.

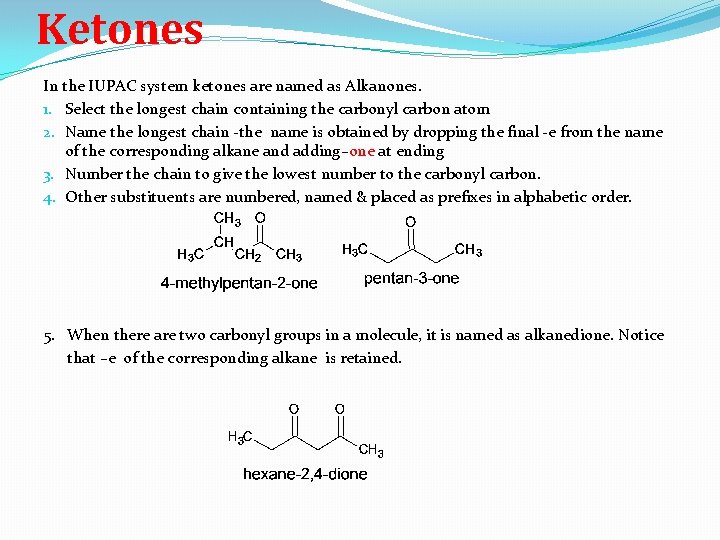
Ketones In the IUPAC system ketones are named as Alkanones. 1. Select the longest chain containing the carbonyl carbon atom 2. Name the longest chain -the name is obtained by dropping the final -e from the name of the corresponding alkane and adding–one at ending 3. Number the chain to give the lowest number to the carbonyl carbon. 4. Other substituents are numbered, named & placed as prefixes in alphabetic order. 5. When there are two carbonyl groups in a molecule, it is named as alkanedione. Notice that –e of the corresponding alkane is retained.

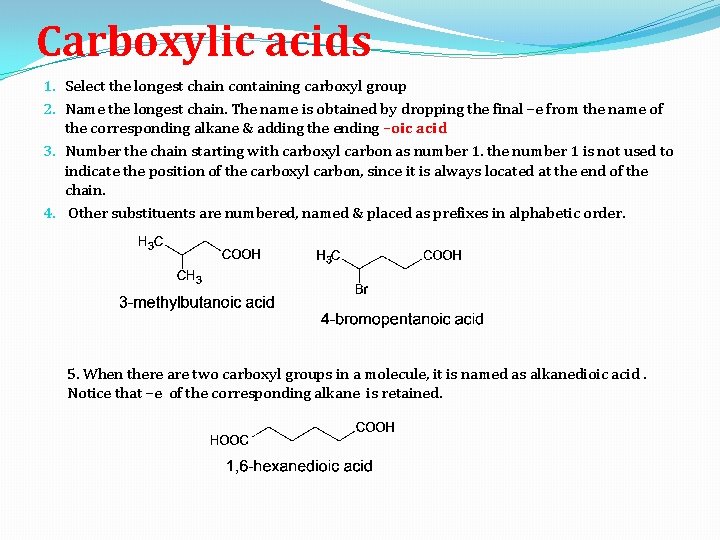
Carboxylic acids 1. Select the longest chain containing carboxyl group 2. Name the longest chain. The name is obtained by dropping the final –e from the name of the corresponding alkane & adding the ending –oic acid 3. Number the chain starting with carboxyl carbon as number 1. the number 1 is not used to indicate the position of the carboxyl carbon, since it is always located at the end of the chain. 4. Other substituents are numbered, named & placed as prefixes in alphabetic order. 5. When there are two carboxyl groups in a molecule, it is named as alkanedioic acid. Notice that –e of the corresponding alkane is retained.

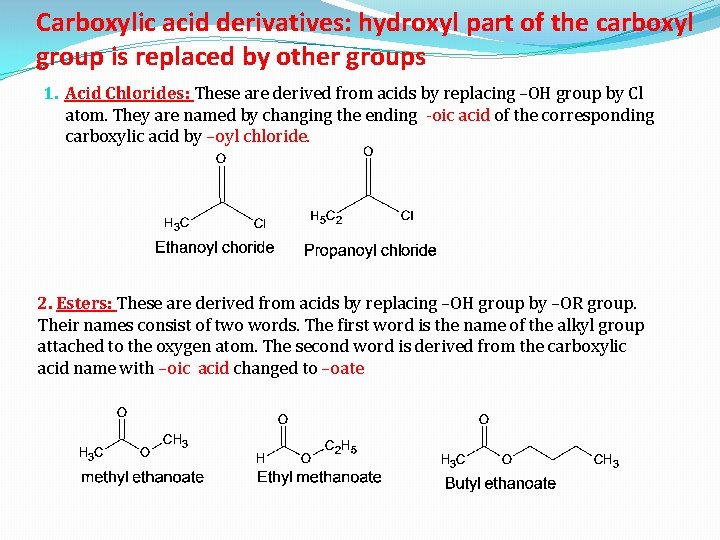
Carboxylic acid derivatives: hydroxyl part of the carboxyl group is replaced by other groups 1. Acid Chlorides: These are derived from acids by replacing –OH group by Cl atom. They are named by changing the ending -oic acid of the corresponding carboxylic acid by –oyl chloride. 2. Esters: These are derived from acids by replacing –OH group by –OR group. Their names consist of two words. The first word is the name of the alkyl group attached to the oxygen atom. The second word is derived from the carboxylic acid name with –oic acid changed to –oate

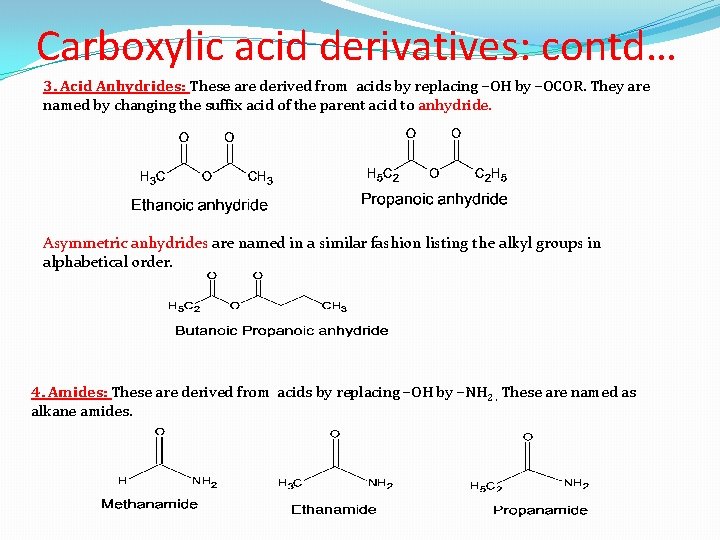
Carboxylic acid derivatives: contd… 3. Acid Anhydrides: These are derived from acids by replacing –OH by –OCOR. They are named by changing the suffix acid of the parent acid to anhydride. Asymmetric anhydrides are named in a similar fashion listing the alkyl groups in alphabetical order. 4. Amides: These are derived from acids by replacing –OH by –NH 2. These are named as alkane amides.

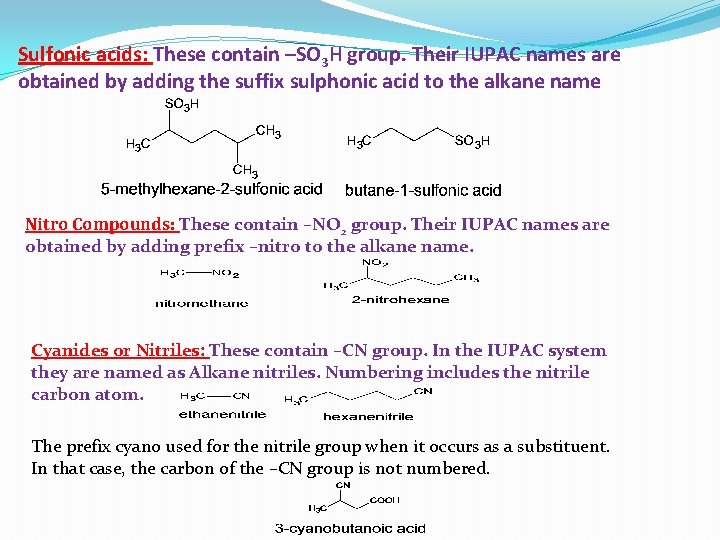
Sulfonic acids: These contain –SO 3 H group. Their IUPAC names are obtained by adding the suffix sulphonic acid to the alkane name Nitro Compounds: These contain –NO 2 group. Their IUPAC names are obtained by adding prefix –nitro to the alkane name. Cyanides or Nitriles: These contain –CN group. In the IUPAC system they are named as Alkane nitriles. Numbering includes the nitrile carbon atom. The prefix cyano used for the nitrile group when it occurs as a substituent. In that case, the carbon of the –CN group is not numbered.

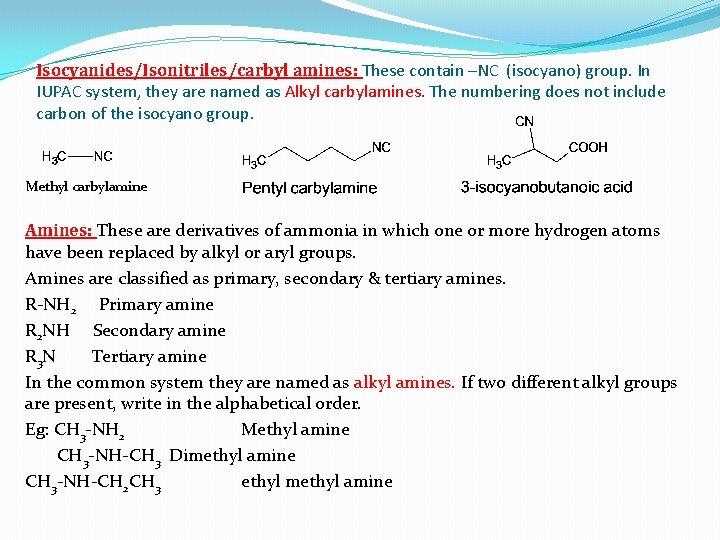
Isocyanides/Isonitriles/carbyl amines: These contain –NC (isocyano) group. In IUPAC system, they are named as Alkyl carbylamines. The numbering does not include carbon of the isocyano group. Methyl carbylamine Amines: These are derivatives of ammonia in which one or more hydrogen atoms have been replaced by alkyl or aryl groups. Amines are classified as primary, secondary & tertiary amines. R-NH 2 Primary amine R 2 NH Secondary amine R 3 N Tertiary amine In the common system they are named as alkyl amines. If two different alkyl groups are present, write in the alphabetical order. Eg: CH 3 -NH 2 Methyl amine CH 3 -NH-CH 3 Dimethyl amine CH 3 -NH-CH 2 CH 3 ethyl methyl amine

Amines: contd…. In the IUPAC system, primary amines are named by replacing the final –e of the parent alkane by –amine. If necessary, a number is added to indicate the position of –NH 2 group. Secondary or tertiary amines are named as N-Substituted derivatives of primary amines. The largest of the groups attached to nitrogen is chosen as the organic group of the primary amine. The remaining alkyl groups are named as substituents by using the prefix N-to indicate that they are attached to nitrogen CH 3 -CH 2 -NH-CH 3 N-Methyl Ethanamine Whenever it is necessary to name –NH 2 as a substituent, it is called the amino group

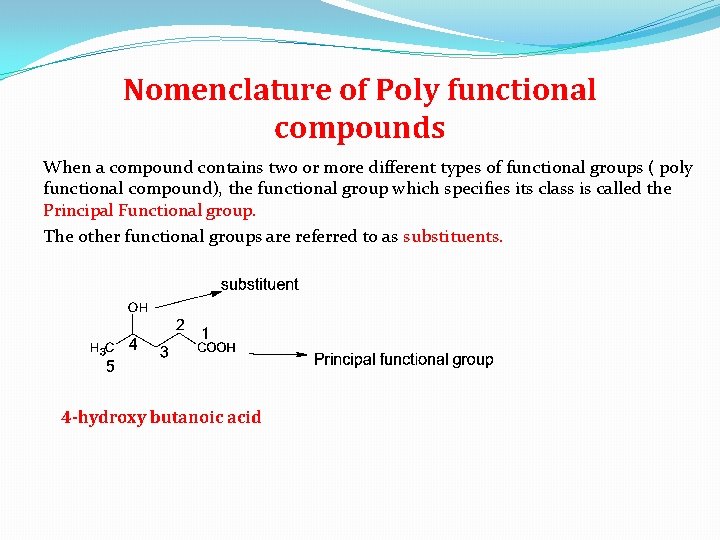
Nomenclature of Poly functional compounds When a compound contains two or more different types of functional groups ( poly functional compound), the functional group which specifies its class is called the Principal Functional group. The other functional groups are referred to as substituents. 4 -hydroxy butanoic acid

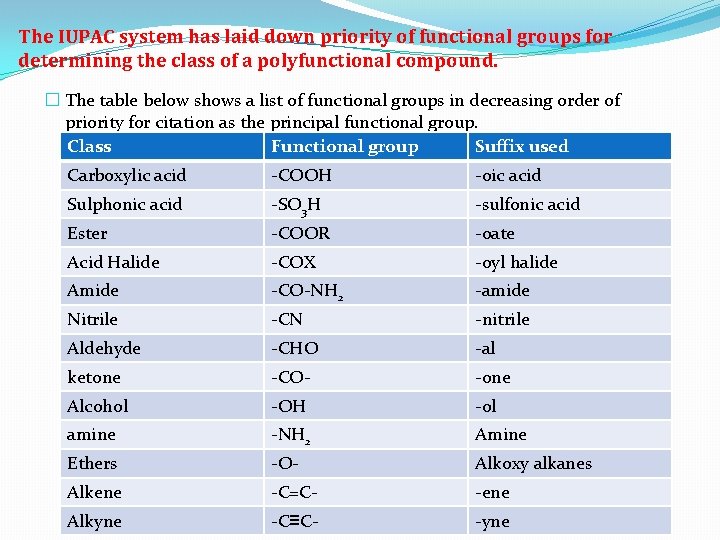
The IUPAC system has laid down priority of functional groups for determining the class of a polyfunctional compound. � The table below shows a list of functional groups in decreasing order of priority for citation as the principal functional group. Class Functional group Suffix used Carboxylic acid -COOH -oic acid Sulphonic acid -SO 3 H -sulfonic acid Ester -COOR -oate Acid Halide -COX -oyl halide Amide -CO-NH 2 -amide Nitrile -CN -nitrile Aldehyde -CHO -al ketone -CO- -one Alcohol -OH -ol amine -NH 2 Amine Ethers -O- Alkoxy alkanes Alkene -C=C- -ene Alkyne -C≡C- -yne

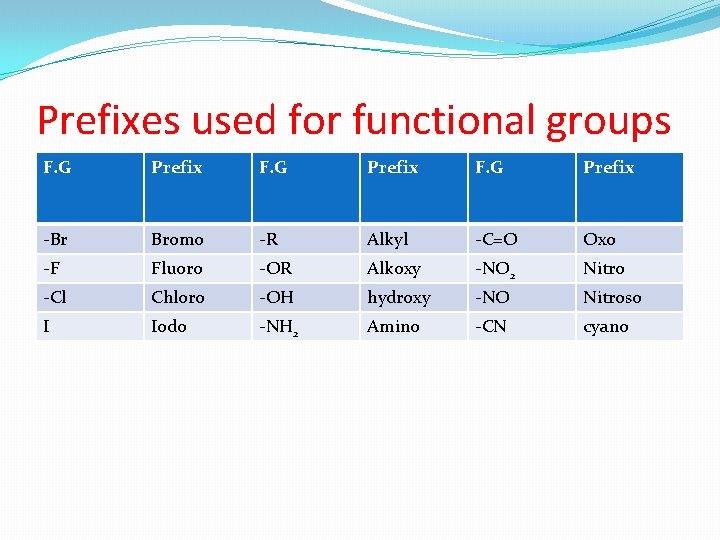
Prefixes used for functional groups F. G Prefix -Br Bromo -R Alkyl -C=O Oxo -F Fluoro -OR Alkoxy -NO 2 Nitro -Cl Chloro -OH hydroxy -NO Nitroso I Iodo -NH 2 Amino -CN cyano

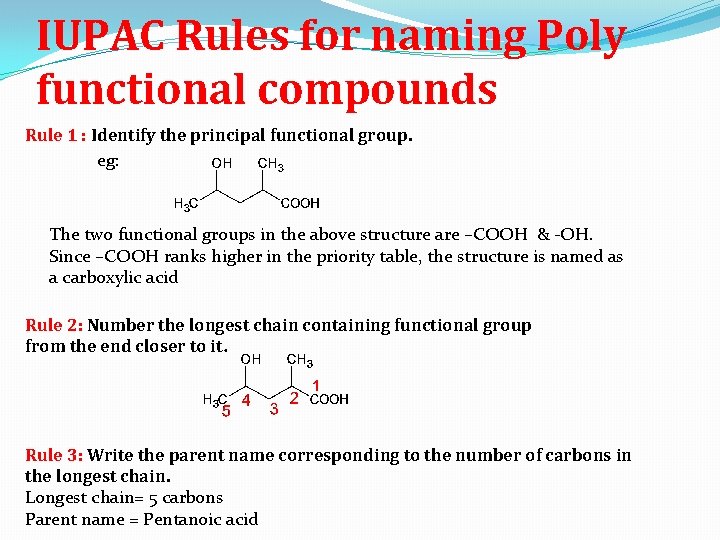
IUPAC Rules for naming Poly functional compounds Rule 1 : Identify the principal functional group. eg: The two functional groups in the above structure are –COOH & -OH. Since –COOH ranks higher in the priority table, the structure is named as a carboxylic acid Rule 2: Number the longest chain containing functional group from the end closer to it. Rule 3: Write the parent name corresponding to the number of carbons in the longest chain. Longest chain= 5 carbons Parent name = Pentanoic acid

contd…. . Rule 4 : Arrange the substituent names with position numbers in alphabetic order 4 -hydroxy-2 -methyl Rule 5 : prefix substituent names with the parent name 4 -hydroxy-2 -methyl pentanoic acid Rule 6: C-C double or triple bonds are usually indicated by integrating –ene or –yne into the suffix, as in –enoic acid or –ynoic acid Rule 7: compounds containing both db & tb in the main chain are named as alkeneynes. The position number of db is inserted before-alken- & that of tb before -yne. Rule 8: The following functional groups are always named as substituents. -X ( all halo groups), -CN, -R, -OR, -NH 2, -NO

Excercises

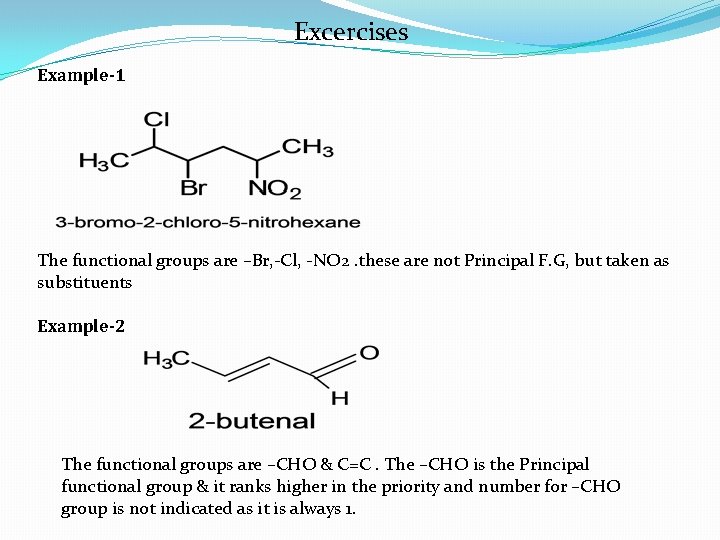
Excercises Example-1 The functional groups are –Br, -Cl, -NO 2. these are not Principal F. G, but taken as substituents Example-2 The functional groups are –CHO & C=C. The –CHO is the Principal functional group & it ranks higher in the priority and number for –CHO group is not indicated as it is always 1.

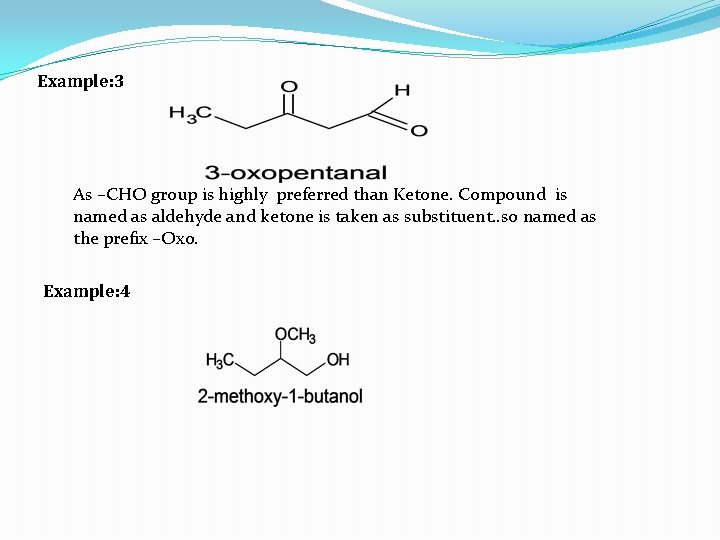
Example: 3 As –CHO group is highly preferred than Ketone. Compound is named as aldehyde and ketone is taken as substituent. . so named as the prefix –Oxo. Example: 4

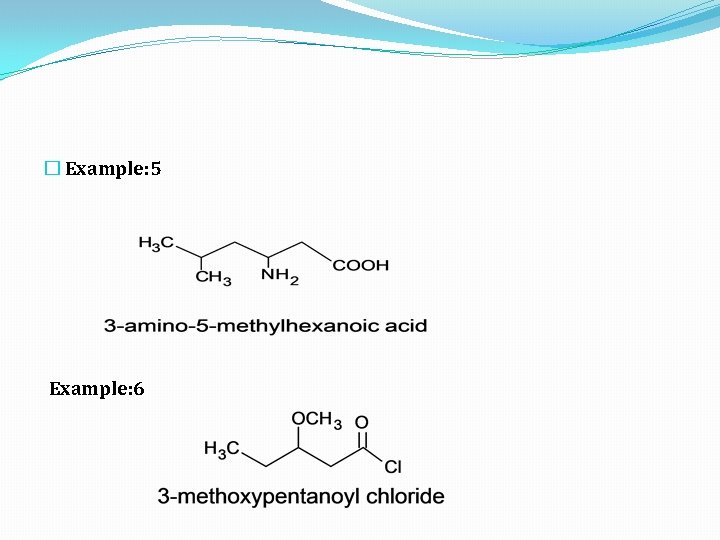
� Example: 5 Example: 6

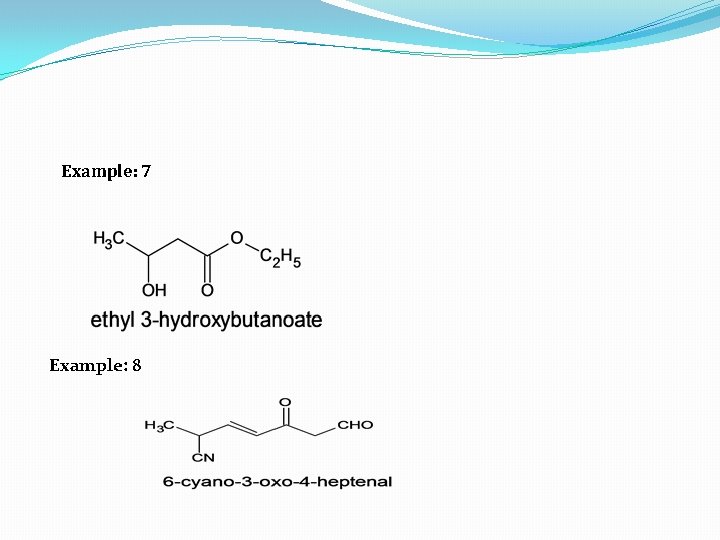
Example: 7 Example: 8

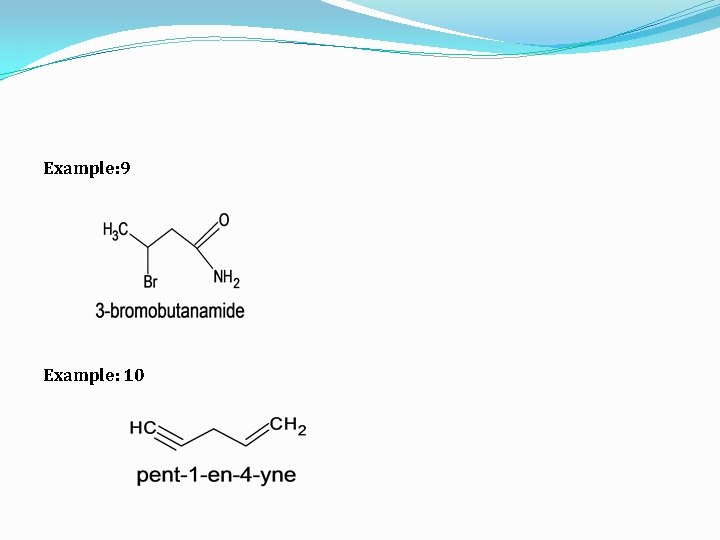
Example: 9 Example: 10


Any queries? ? ? ?


THANK YOU
 Ch3ch2ch2cooch(ch3)2 name
Ch3ch2ch2cooch(ch3)2 name Jyothi singh
Jyothi singh Jeeva jyothi
Jeeva jyothi Alkene alcohol naming
Alkene alcohol naming Organic chemistry nomenclature
Organic chemistry nomenclature Basic organic nomenclature packet
Basic organic nomenclature packet Nomenclature of heterocyclic compounds
Nomenclature of heterocyclic compounds Nomenclature of binary ionic compounds
Nomenclature of binary ionic compounds Nomenclature of coordination compounds
Nomenclature of coordination compounds Heterocyclic compounds nomenclature
Heterocyclic compounds nomenclature Organic vs inorganic compounds
Organic vs inorganic compounds Is hagfish slime a lipid
Is hagfish slime a lipid Classification of hydrocarbons
Classification of hydrocarbons Vitamins are organic compounds
Vitamins are organic compounds Pyranoses
Pyranoses What is the difference between organic and inorganic
What is the difference between organic and inorganic Meaning of the word organic
Meaning of the word organic Four types
Four types Quantitative analysis of organic compounds ppt
Quantitative analysis of organic compounds ppt What is the classification of organic compounds
What is the classification of organic compounds All organic compounds must contain the element
All organic compounds must contain the element Celiac beri beri
Celiac beri beri Metabolic changes of drugs and related organic compounds
Metabolic changes of drugs and related organic compounds Organic compounds grade 10 life science
Organic compounds grade 10 life science Purification and characterization of organic compounds
Purification and characterization of organic compounds Biochemistry
Biochemistry Decomposition of organic matter equation
Decomposition of organic matter equation Type of organic compounds
Type of organic compounds Carbon compound
Carbon compound Organic compounds must contain:
Organic compounds must contain: What is a nucleotide
What is a nucleotide Are vitamins tasteless
Are vitamins tasteless All organic compounds contain carbon and ________.
All organic compounds contain carbon and ________. Organic halogen compounds
Organic halogen compounds Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Burning of organic compounds
Burning of organic compounds Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Sextext of electrons
Sextext of electrons Principle of distillation
Principle of distillation Organic vs inorganic compounds
Organic vs inorganic compounds Cho
Cho Are vitamins organic compounds
Are vitamins organic compounds Families of organic compounds
Families of organic compounds Venn diagram ionic and covalent bonds
Venn diagram ionic and covalent bonds General chemistry nomenclature
General chemistry nomenclature Rh nomenclature
Rh nomenclature Nomenclature eolienne
Nomenclature eolienne What is classifying
What is classifying Unsaturated hydrocarbon
Unsaturated hydrocarbon What does na2cr2o7 do
What does na2cr2o7 do Naming alkynes
Naming alkynes Quiz 2 binomial nomenclature
Quiz 2 binomial nomenclature Example of criminal law
Example of criminal law Scientific taxonomy
Scientific taxonomy Non essential fatty acids
Non essential fatty acids Nomenclature acide carboxylique
Nomenclature acide carboxylique Ieee nomenclature
Ieee nomenclature