NOMENCLATURE OF INORGANIC COMPOUNDS v NOMENCLATURE Nomen Name










- Slides: 17

NOMENCLATURE OF INORGANIC COMPOUNDS v NOMENCLATURE : Nomen = Name Calare = to call v CHEMICAL NOMENCLATURE is the naming of substances v INORGANIC COMPOUNDS composed of elements other than carbon. Associated with the nonliving portion of the world.

NOMENCLATURE OF INORGANIC COMPOUNDS v Ionic compounds v Molecular compunds v Acids

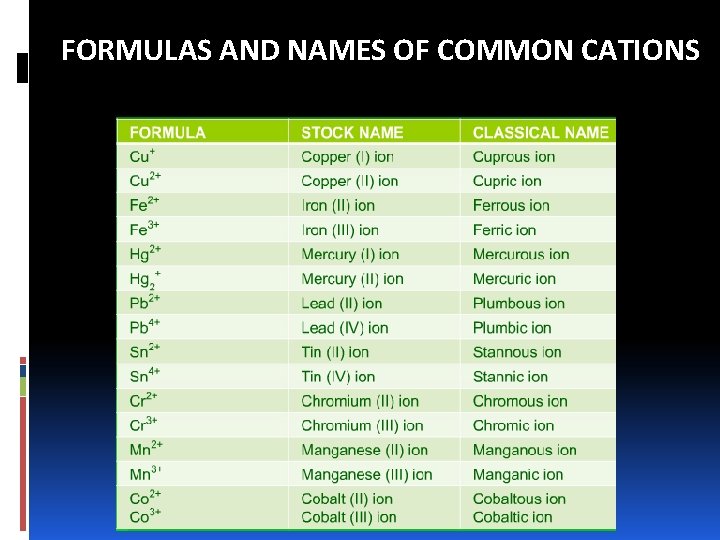
NAMING IONIC COMPOUNDS: CATIONS A. Cations made up of metal atoms have the same name as the metal. Na+ = sodium ion Zn 2+ = zinc ion Al 3+ = aluminum ion B. If a metal can form cations of different charges, the positive charge is given by roman numbers into brackets following the name of the metal Fe 2+ = iron (II) ion Cu+ = copper (I) ion Fe 3+ = iron (III) ion Cu 2+ = copper (II) iron

NAMING IONIC COMPOUNDS: CATIONS v Ions with different charges exhibit different properties, such as color. v Most of the metals that have variable charges are transition metals. v Applying –ous and –ic. The endings represent the lower and higher charged ions, respectively. Fe 2+ = ferrous ion Cu+ = cuprous ion Fe 3+ = ferric ion Cu 2+ = cupric iron

NAMING IONIC COMPOUNDS: CATIONS C. Cations formed from nonmetals have names that end in -ium. NH 4+ = ammonium ion H 3 O+ = hydronium ion

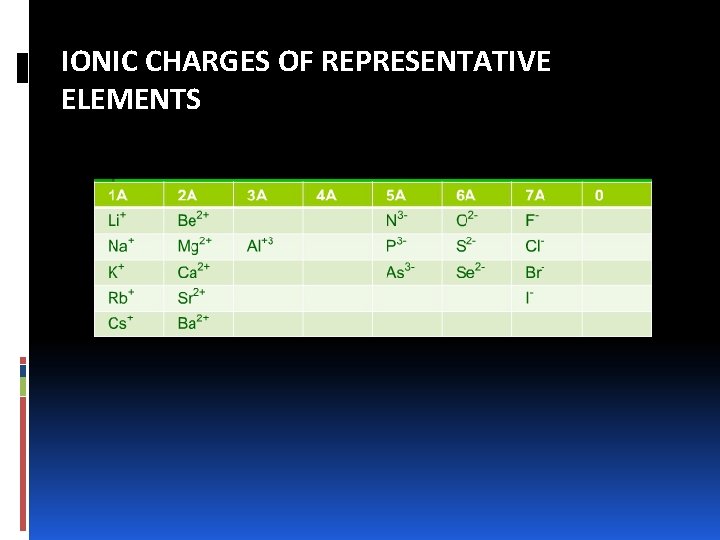
IONIC CHARGES OF REPRESENTATIVE ELEMENTS

FORMULAS AND NAMES OF COMMON CATIONS

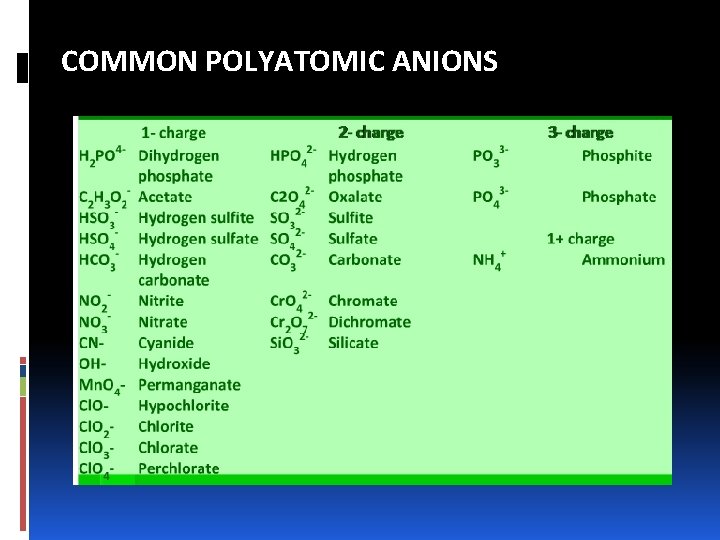
NAMING IONIC COMPOUNDS: ANIONS A. Mono atomic anions have names formed by replacing the ending of the name of the element with -ide H- = hydride ion O 2 - = oxide ion N 3 - = nitride ion B. Poly atomic anions containing oxygen have names ending in –ate or –ite. These are called oxyanions. NO 3 - = nitrate ion SO 42 - = sulfate ion NO 2 - = nitrite ion SO 32 - = sulfite ion

NAMING IONIC COMPOUNDS: ANIONS v Prefixes are used when the series of oxyanions of an element extends to four members, as with halogens. § Cl. O 4 - = perchlorate ion, Cl (VII+) § Cl. O 3 - = chlorate ion, Cl (V+) § Cl. O 2 - = chlorite ion, Cl (III+) § Cl. O- = hypochlorite ion, Cl (I+) v The prefix per- indicates one or more oxygen atoms than the oxyanion ending in –ate. SO 52 - = peroxomonosulfate ion

NAMING IONIC COMPOUNDS: ANIONS C. Anions derived by adding a H to an oxyanion are named by adding as a prefix the word hydrogen or dihydrogen. CO 32 - = carbonate ion HCO 3 - = hydrogen carbonate ion PO 43 - = phosphate ion H 2 PO 4 - = dihydrogen phopsphate ion

COMMON POLYATOMIC ANIONS

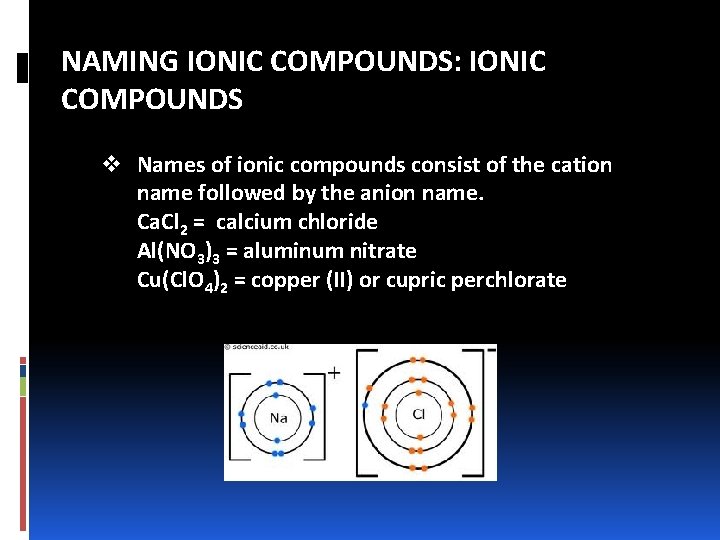
NAMING IONIC COMPOUNDS: IONIC COMPOUNDS v Names of ionic compounds consist of the cation name followed by the anion name. Ca. Cl 2 = calcium chloride Al(NO 3)3 = aluminum nitrate Cu(Cl. O 4)2 = copper (II) or cupric perchlorate

NAMING IONIC COMPOUNDS: ACIDS v An acid is a substance whose molecules yield hydrogen ions (H+) when dissolved in water (BrØnsted-Lowry).

NAMING IONIC COMPOUNDS: ACIDS v Acid based on anions whose names end in –ide have associated acids that have the hydro- prefix and –ic ending. Cl- → HCl(aq) = hydrochloric acid S 2 - → H 2 S(aq) = hydrosulfuric acid

NAMING IONIC COMPOUNDS: ACIDS v Acid based on anions whose names end in –ate have associated acids with an –ic ending, whereas anions whose names end in –ite are related to acids with an –ous ending. Prefixes in the name of the anion are retained in the name of the acid. Cl. O 4 - = perchlorate ion → HCl. O 4 = perchloric acid Cl. O 3 - = chlorate ion → HCl. O 3 = chloric acid Cl. O 2 - = chlorite ion → HCl. O 2 = chlorous acid Cl. O- = hypochlorite ion → HCl. O = hypochlorous acid

NAMING NON IONIC COMPOUNDS v Similar rules to those used for naming ionic compounds. v The name of the element farthest to the left in the periodic table is usually written first. EXCEPTION: compounds that contain oxygen, oxygen always written last. v If both elements are in the same group in the periodic table, the lower one is named first. v The name of the second element is given an –ide ending.

NAMING NON IONIC COMPOUNDS v Greek prefixes are used to indicate the number of atoms of each element. v Mono- is never used with the first element. v The prefix ends in a or o, if the name of the second element begins with a vowel, the a or o drops. Cl 2 O = dichlorine monoxide NF 3 = nitrogen trifluoride N 2 O 4 = dinitrogen tretoxide. P 4 S 10 = tetraphosphorous decasulfide