Nomenclature of Inorganic Compounds Chapter 6 Outline I
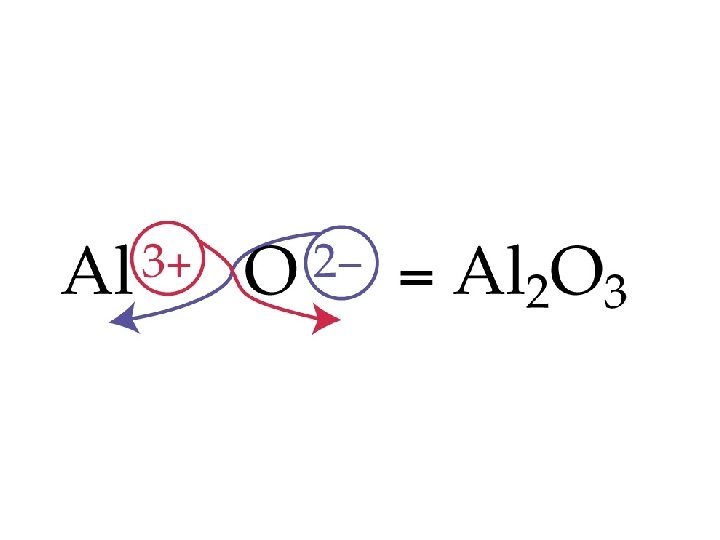
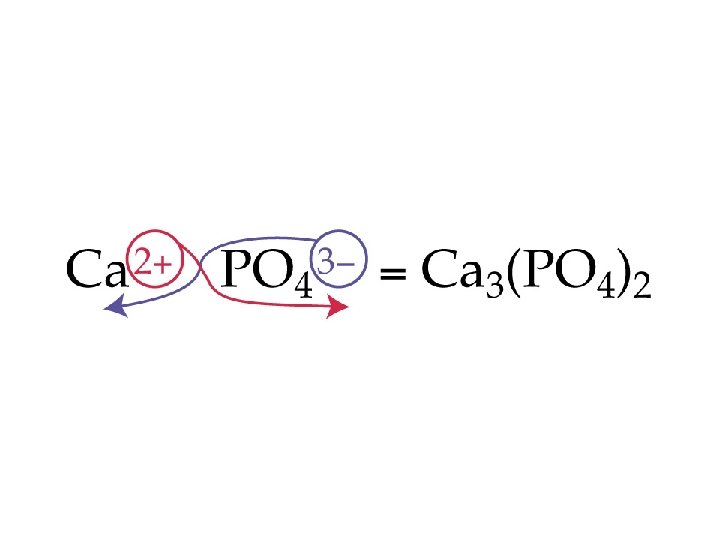











- Slides: 50

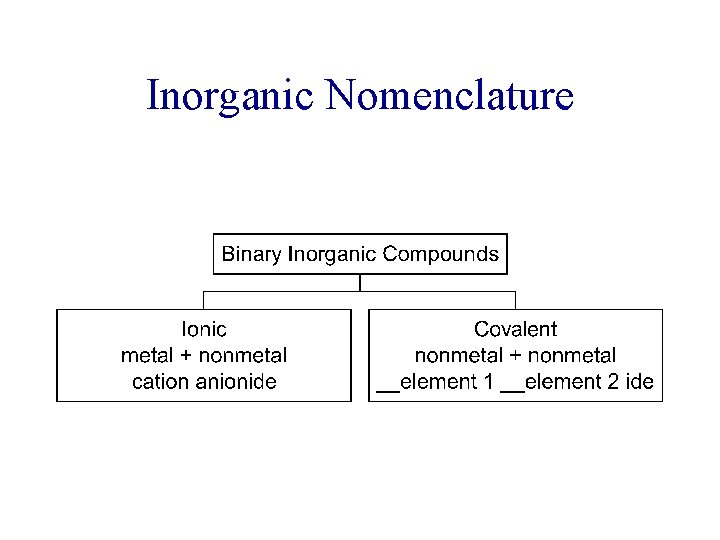
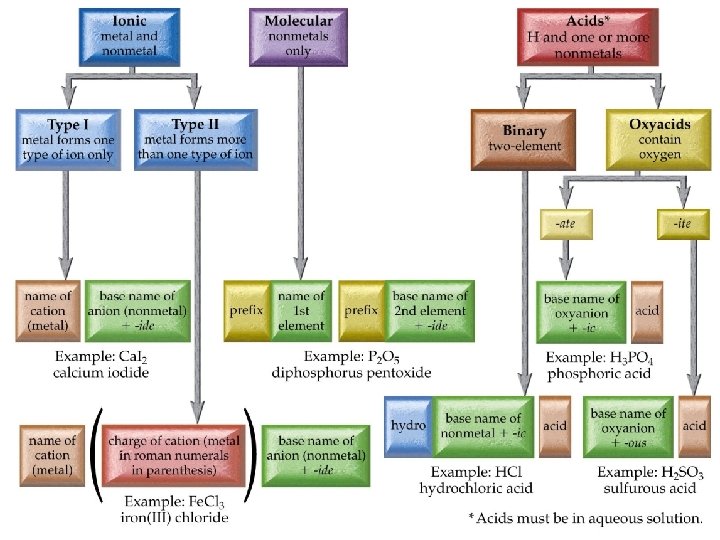
Nomenclature of Inorganic Compounds Chapter 6 Outline I. Octet Rule II. Ionic Compounds A. Binary Nomenclature B. Polyatomic Nomenclature III. Covalent Compounds A. Binary Nomenclature IV. Acids A. Binary Nomenclature B. Ternary Nomenclature (Oxyacids) V. Hydrates


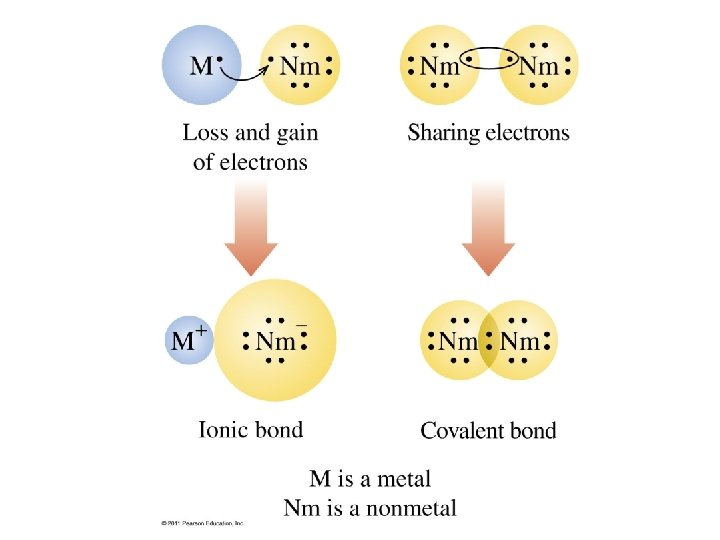
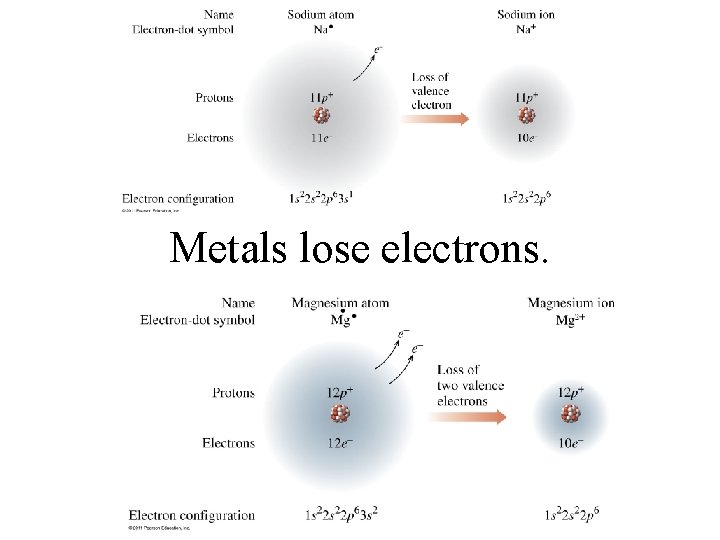
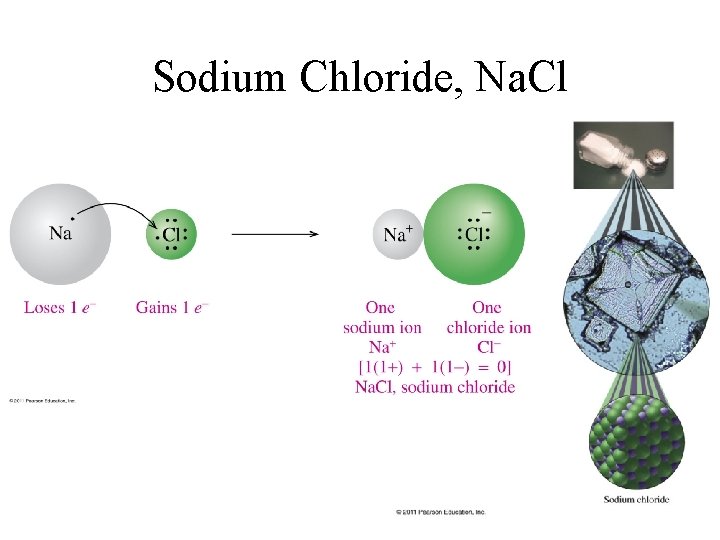
Metals lose electrons.

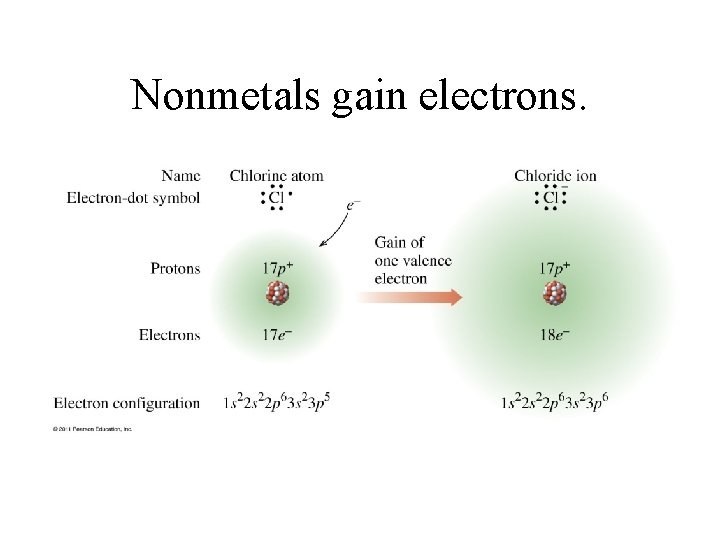
Nonmetals gain electrons.

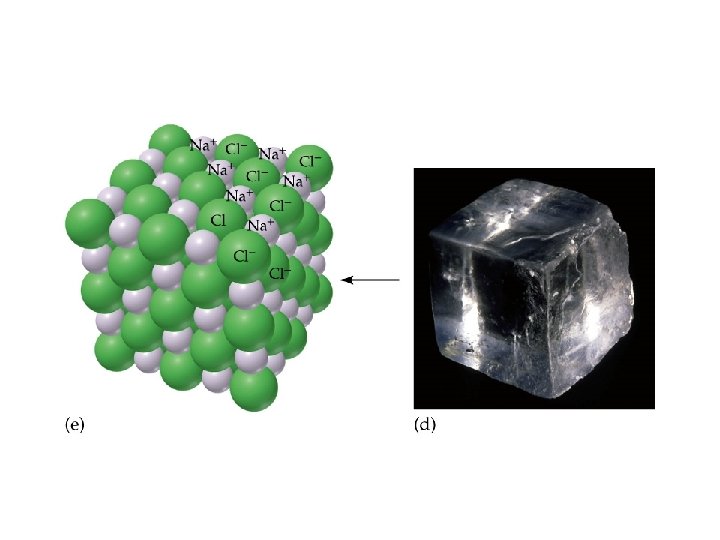
Sodium Chloride, Na. Cl

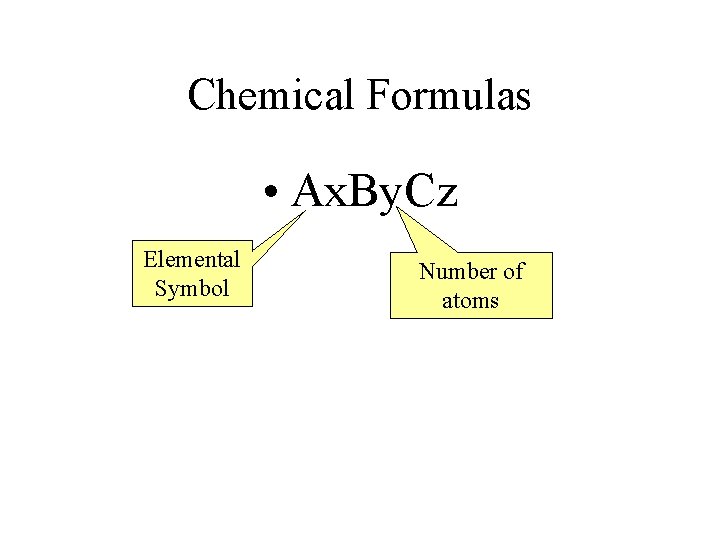
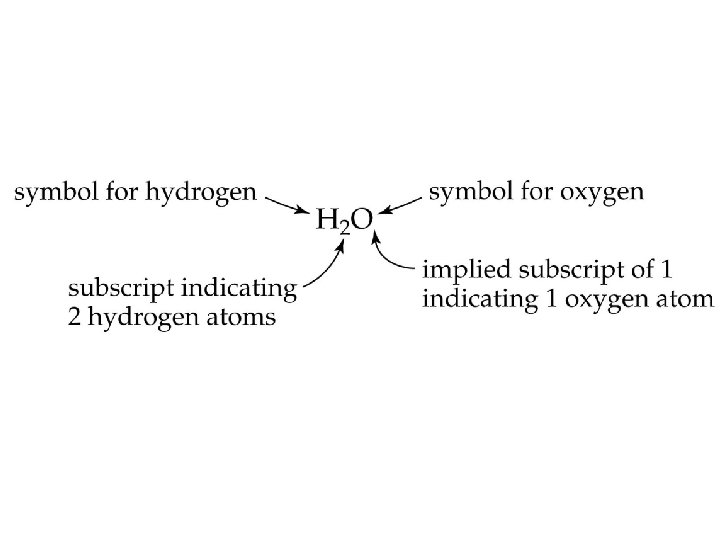
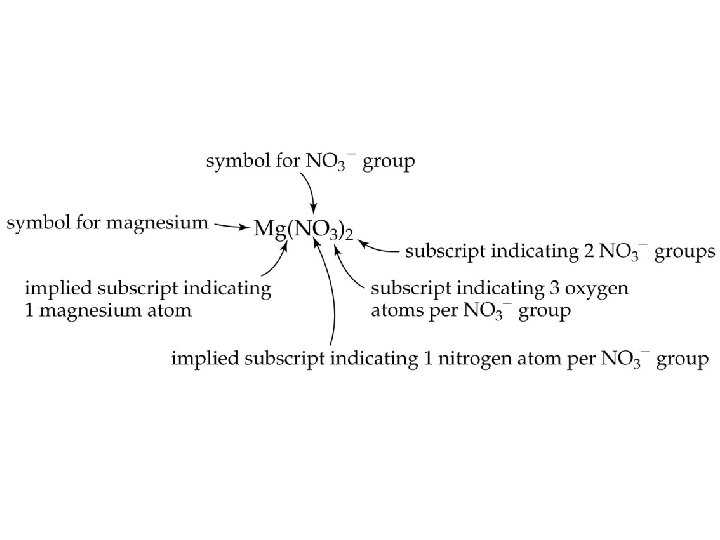
Chemical Formulas • Ax. By. Cz Elemental Symbol Number of atoms



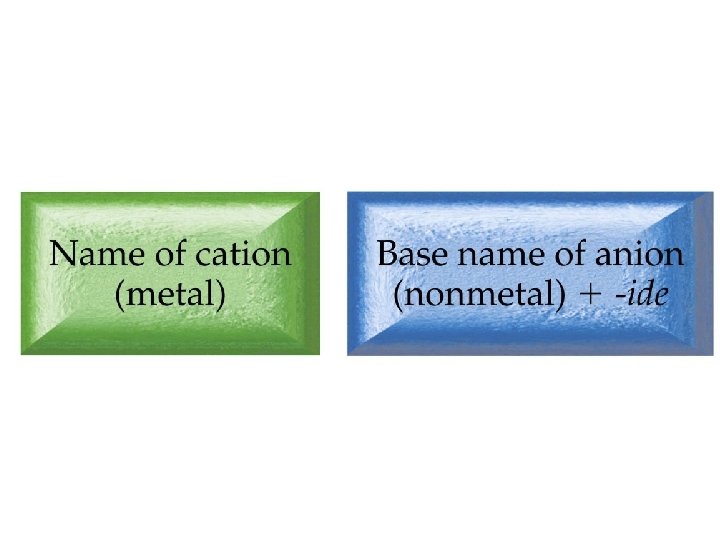
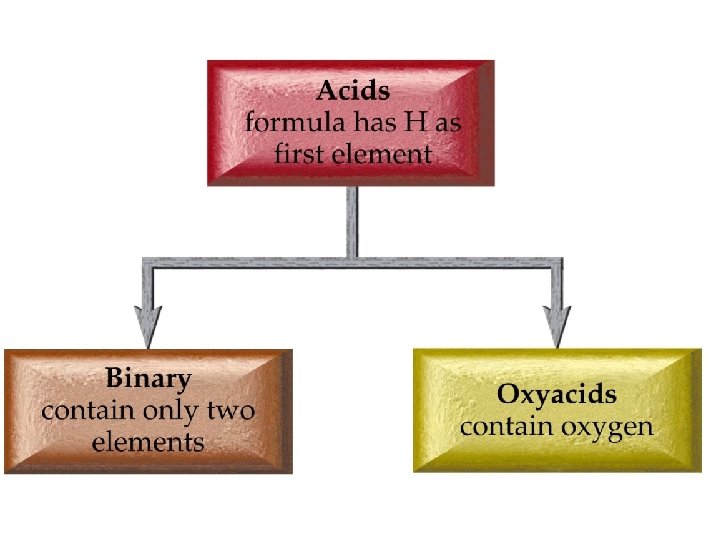
Inorganic Nomenclature

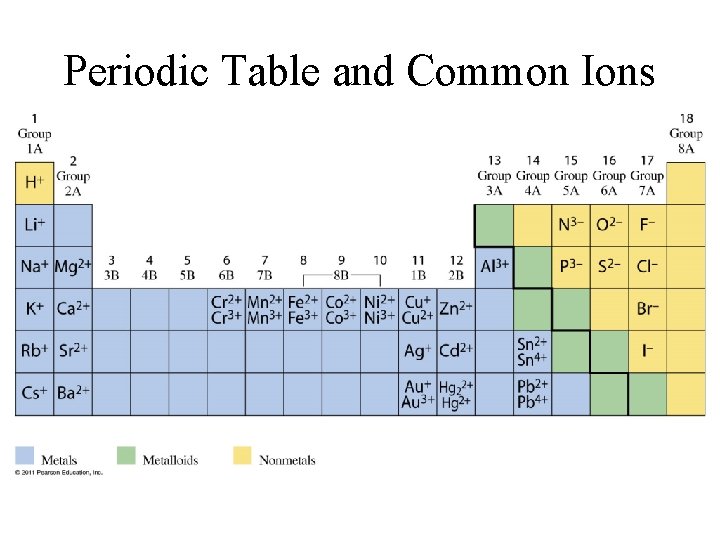
Periodic Table and Common Ions
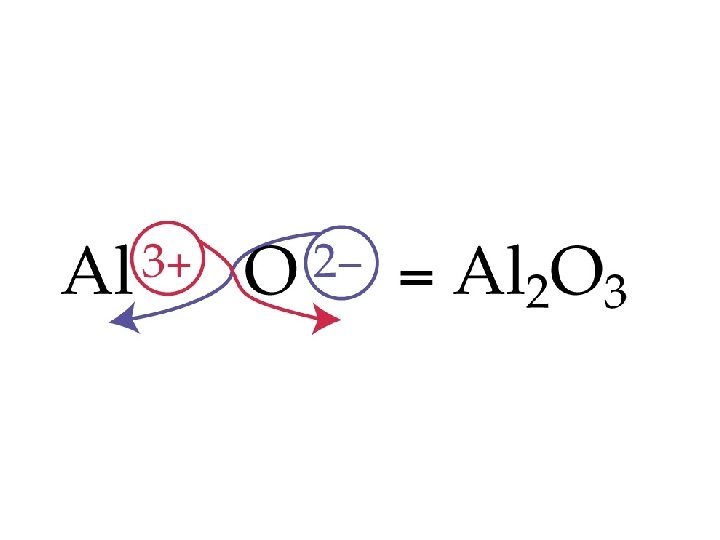
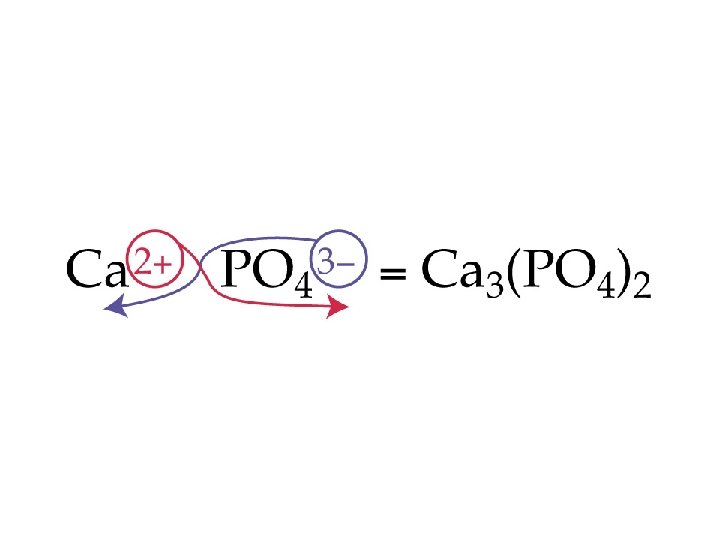





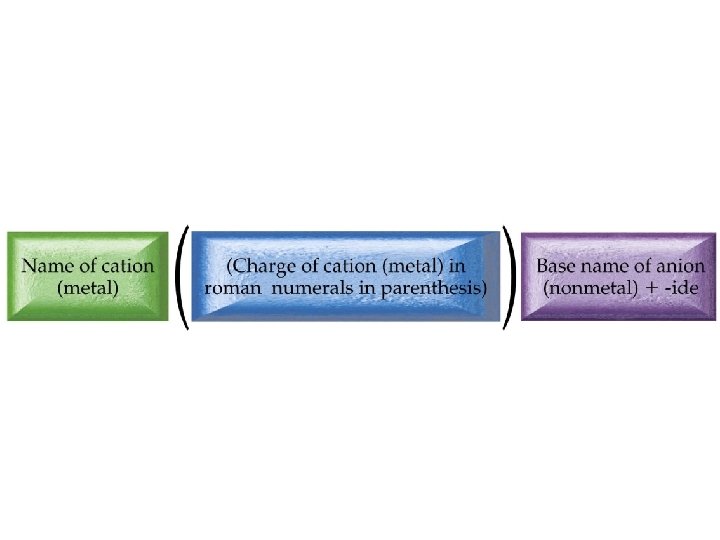
Latin names for cations • • • Copper(I) = cuprous = Cu+ Copper(II) = cupric = Cu 2+ Iron(II) = ferrous = Fe 2+ Iron(III) = ferric Fe 3+ Tin(II) = stannous = Sn 2+ Tin(IV) = stannic = Sn 4+

Mercury • Remember— • Mercury(I) exists only as Hg 22+ • Mercury(II) is Hg 2+

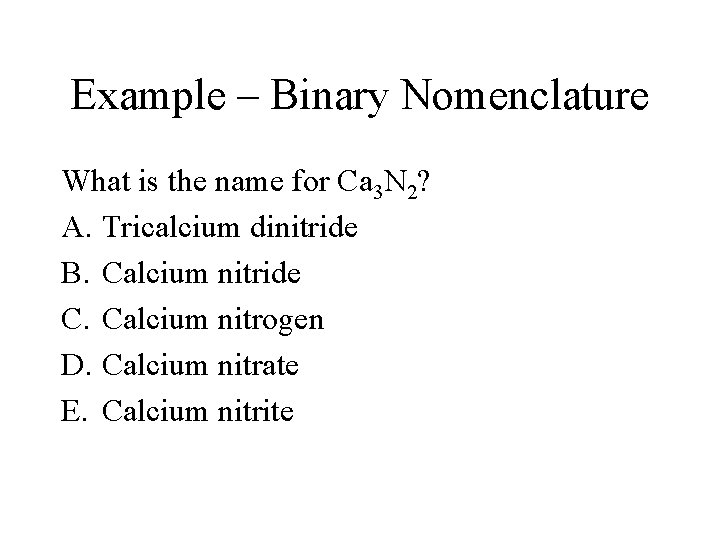
Example – Binary Nomenclature What is the name for Ca 3 N 2? A. Tricalcium dinitride B. Calcium nitride C. Calcium nitrogen D. Calcium nitrate E. Calcium nitrite

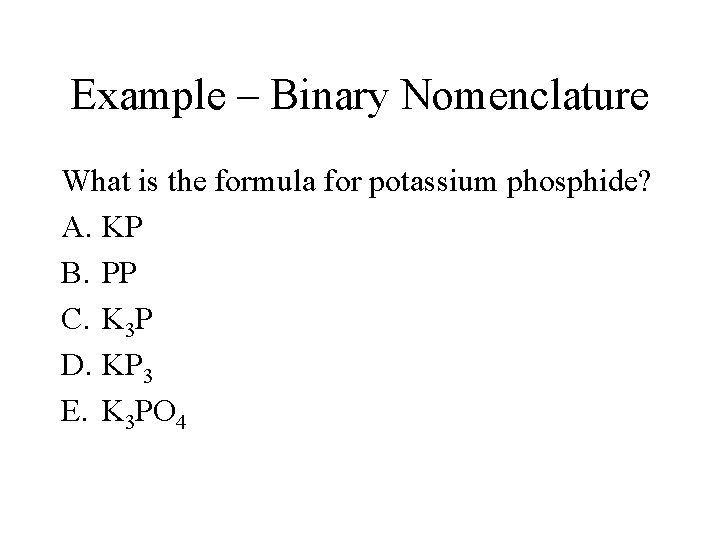
Example – Binary Nomenclature What is the formula for potassium phosphide? A. KP B. PP C. K 3 P D. KP 3 E. K 3 PO 4

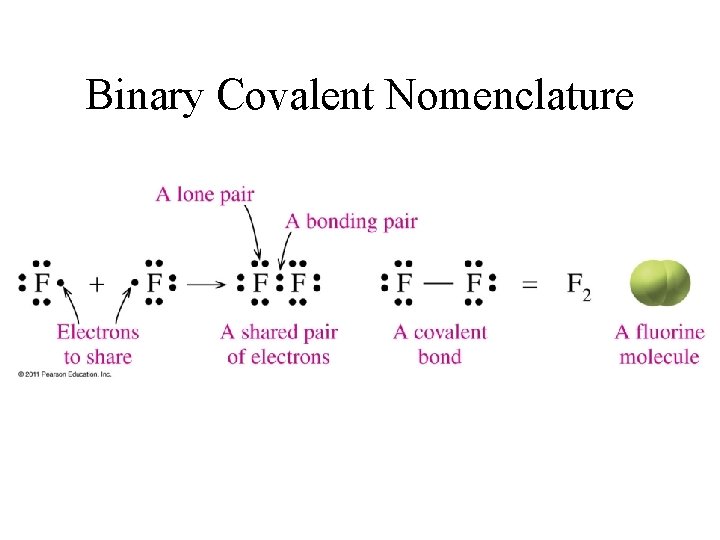
Binary Covalent Nomenclature

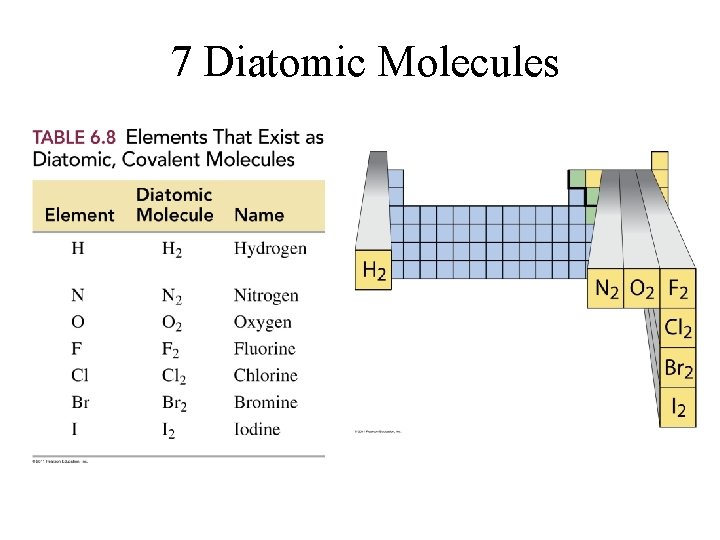
7 Diatomic Molecules

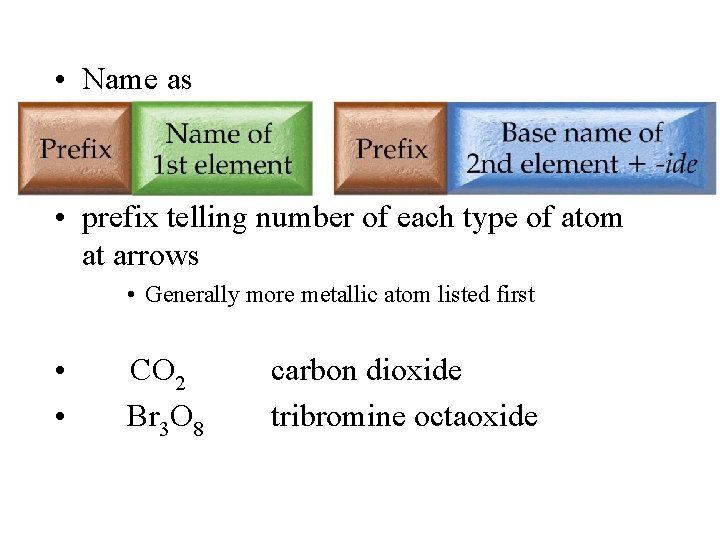
• Name as • _____ element 1 _____ element 2 ide • prefix telling number of each type of atom at arrows • Generally more metallic atom listed first • • CO 2 Br 3 O 8 carbon dioxide tribromine octaoxide

Prefixes • • • mono di tri tetra penta 1 2 3 4 5 • • • hexa hepta octa nona deca 6 7 8 9 10

Example – Binary Nomenclature What is the formula for dinitrogen monoxide? A. 2 NO B. N 2 O C. NO 2 D. N 2 O 2 E. NO

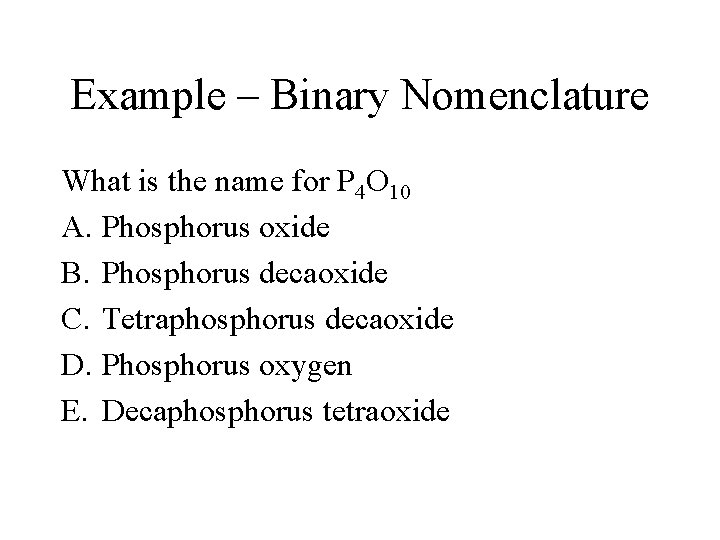
Example – Binary Nomenclature What is the name for P 4 O 10 A. Phosphorus oxide B. Phosphorus decaoxide C. Tetraphosphorus decaoxide D. Phosphorus oxygen E. Decaphosphorus tetraoxide

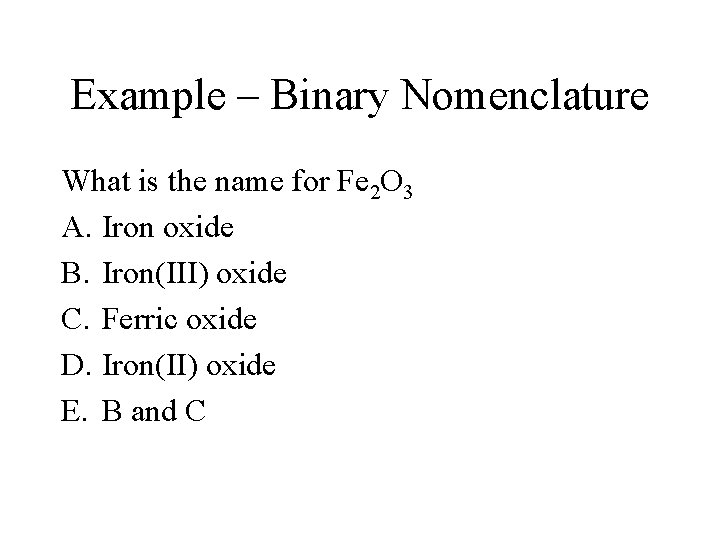
Example – Binary Nomenclature What is the name for Fe 2 O 3 A. Iron oxide B. Iron(III) oxide C. Ferric oxide D. Iron(II) oxide E. B and C

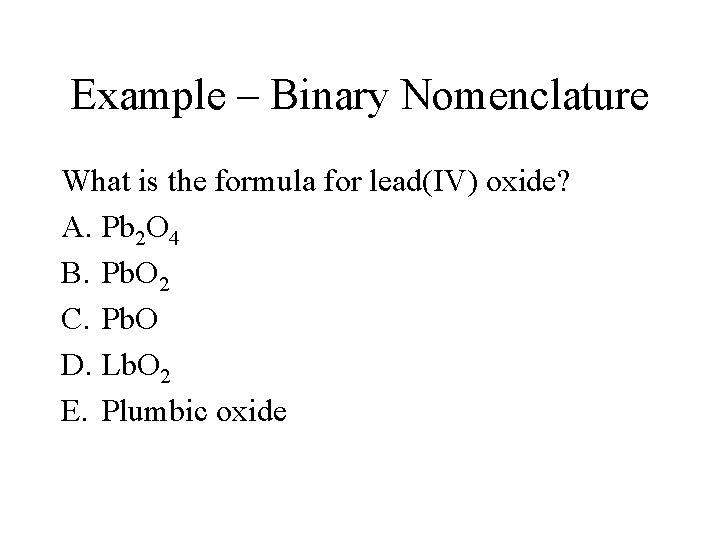
Example – Binary Nomenclature What is the formula for lead(IV) oxide? A. Pb 2 O 4 B. Pb. O 2 C. Pb. O D. Lb. O 2 E. Plumbic oxide


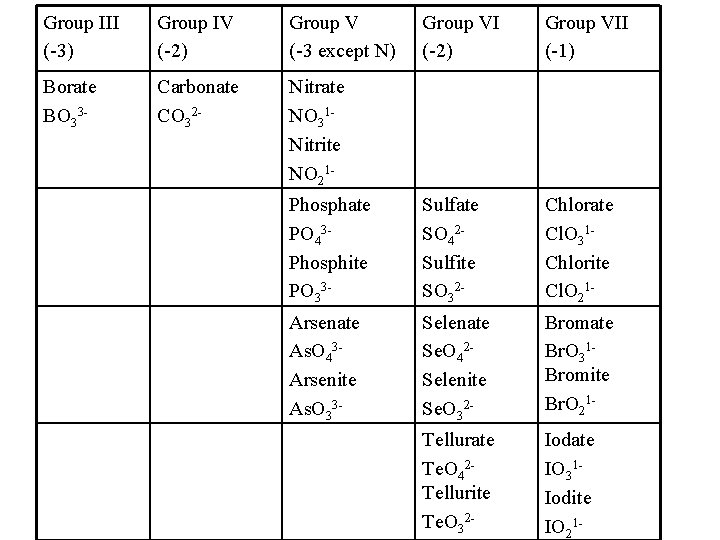
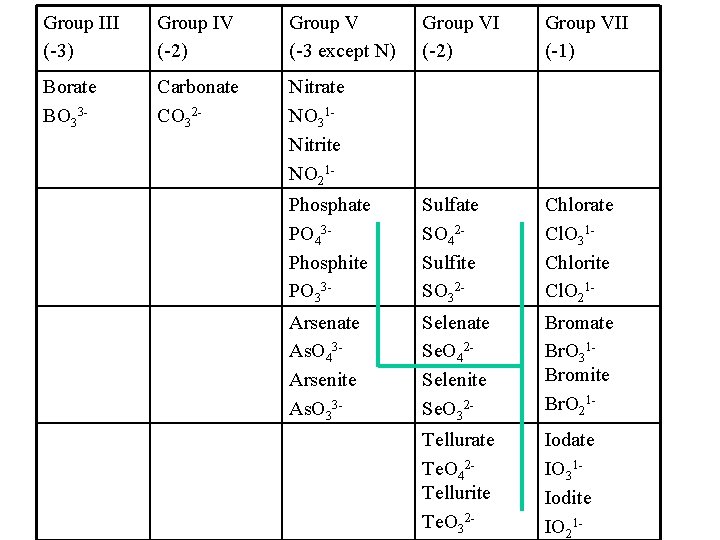
Group III (-3) Group IV (-2) Group V (-3 except N) Group VI (-2) Group VII (-1) Borate BO 33 - Carbonate CO 32 - Nitrate NO 31 Nitrite NO 21 Phosphate PO 43 Phosphite PO 33 - Sulfate SO 42 Sulfite SO 32 - Chlorate Cl. O 31 Chlorite Cl. O 21 - Arsenate As. O 43 Arsenite As. O 33 - Selenate Se. O 42 Selenite Se. O 32 - Bromate Br. O 31 Bromite Br. O 21 - Tellurate Te. O 42 Tellurite Te. O 32 - Iodate IO 31 Iodite IO 21 -

Group III (-3) Group IV (-2) Group V (-3 except N) Group VI (-2) Group VII (-1) Borate BO 33 - Carbonate CO 32 - Nitrate NO 31 Nitrite NO 21 Phosphate PO 43 Phosphite PO 33 - Sulfate SO 42 Sulfite SO 32 - Chlorate Cl. O 31 Chlorite Cl. O 21 - Arsenate As. O 43 Arsenite As. O 33 - Selenate Se. O 42 Selenite Se. O 32 - Bromate Br. O 31 Bromite Br. O 21 - Tellurate Te. O 42 Tellurite Te. O 32 - Iodate IO 31 Iodite IO 21 -


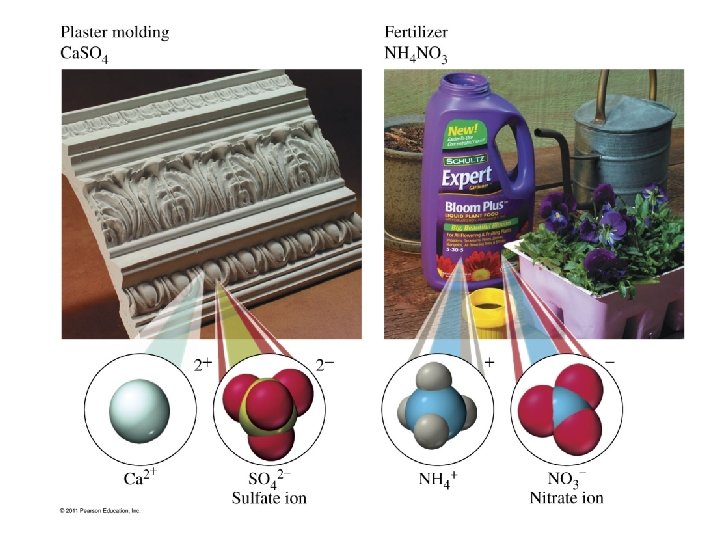
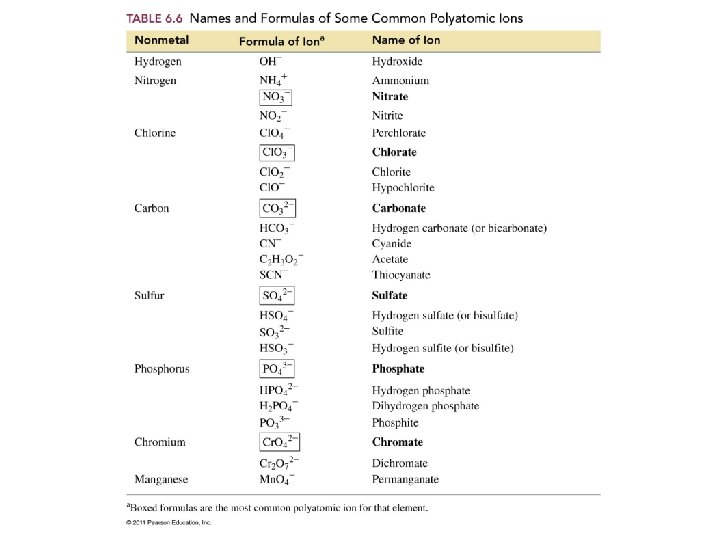
Ions to Memorize • • • NH 41+ ammonium ion OCN 1 - cyanate ion Cr. O 42 - chromate ion Cr 2 O 72 - dichromate ion Mn. O 41 - permanganate ion NO 31 - nitrate ion • • • CN 1 C 2 H 3 O 21 C 2 O 42 OH 1 O 22 - cyanide ion acetate ion oxalate ion hydroxide ion peroxide ion

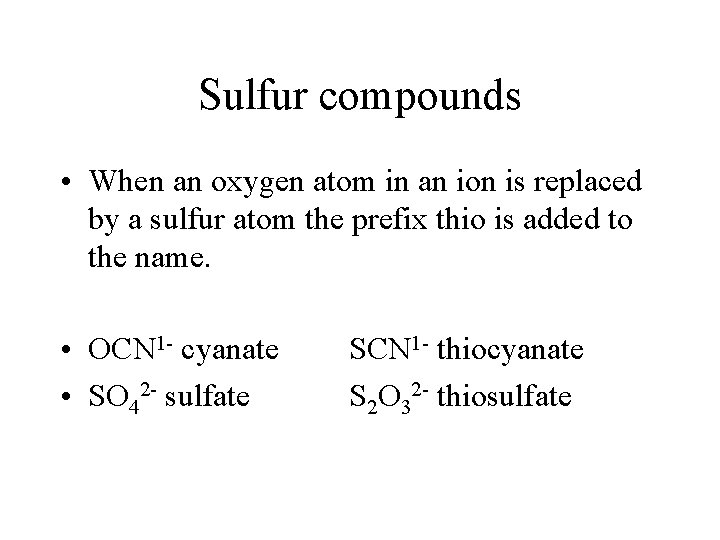
Sulfur compounds • When an oxygen atom in an ion is replaced by a sulfur atom the prefix thio is added to the name. • OCN 1 - cyanate • SO 42 - sulfate SCN 1 - thiocyanate S 2 O 32 - thiosulfate


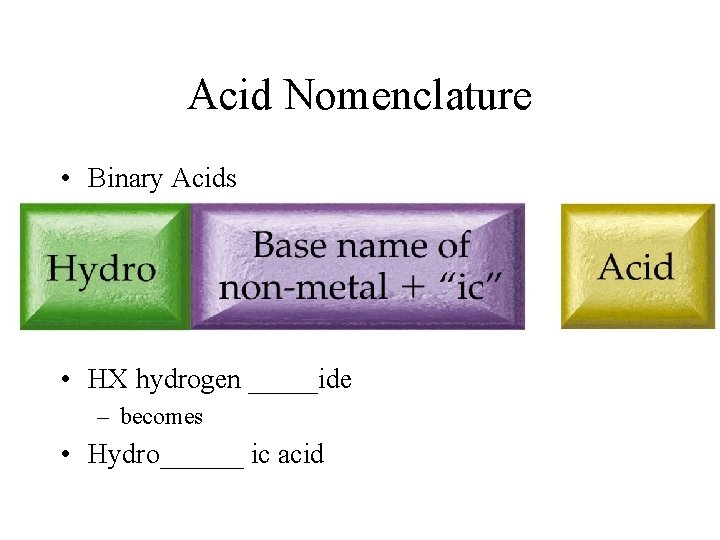
Acid Nomenclature • Binary Acids • HX hydrogen _____ide – becomes • Hydro______ ic acid

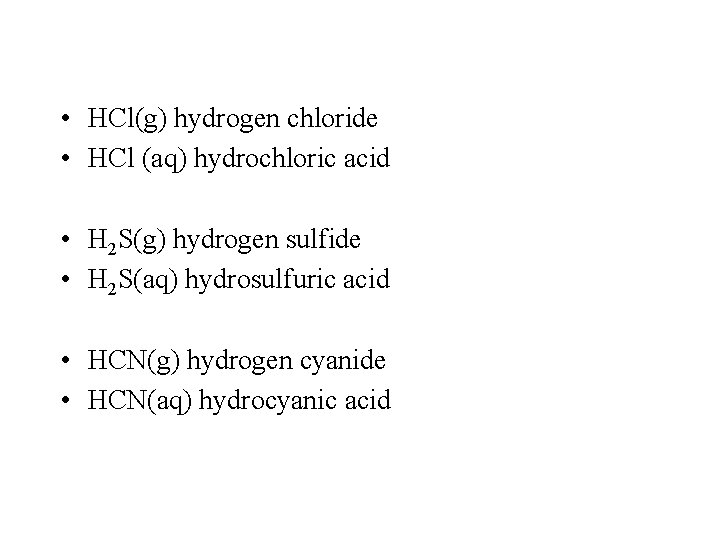
• HCl(g) hydrogen chloride • HCl (aq) hydrochloric acid • H 2 S(g) hydrogen sulfide • H 2 S(aq) hydrosulfuric acid • HCN(g) hydrogen cyanide • HCN(aq) hydrocyanic acid


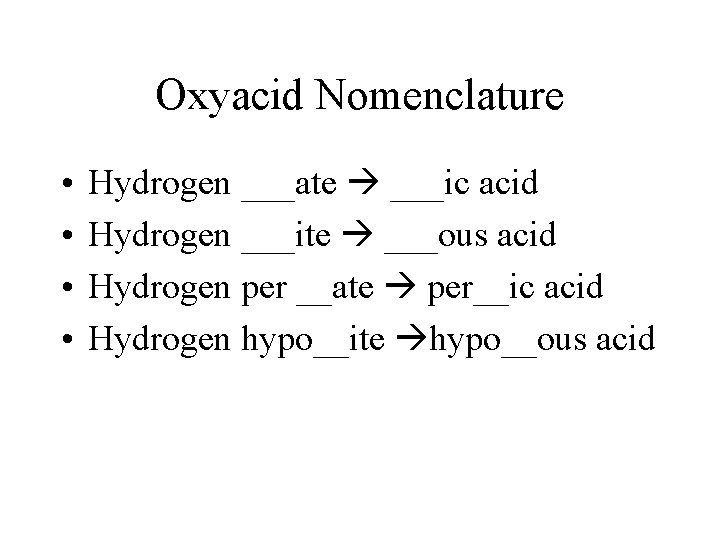
Oxyacid Nomenclature • • Hydrogen ___ate ___ic acid Hydrogen ___ite ___ous acid Hydrogen per __ate per__ic acid Hydrogen hypo__ite hypo__ous acid

Acid salt nomenclature • HX-? Hydrogen anion name • H 2 X-? Dihydrogen anion name • Sometimes use the prefix bi to indicate the presence of one H attached to an anion

• HCO 31 • HS 1 - hydrogen carbonate or bicarbonate hydrogen sulfide or bisulfide • HSO 41 - hydrogen sulfate or bisulfate

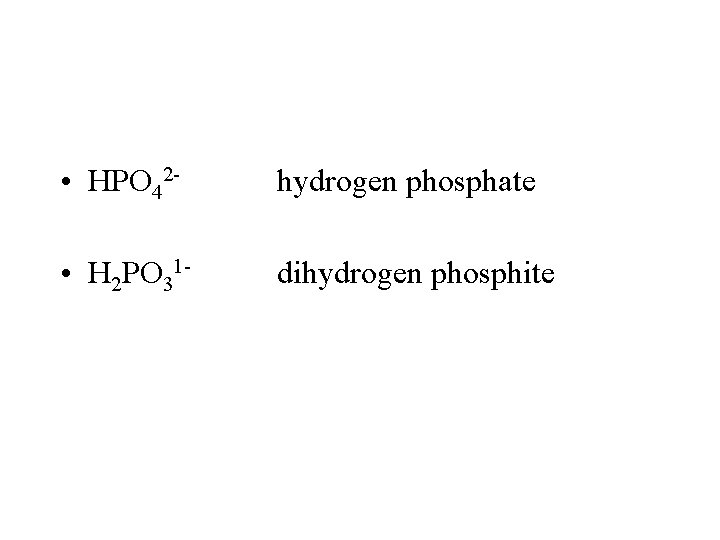
• HPO 42 - hydrogen phosphate • H 2 PO 31 - dihydrogen phosphite


Example – Ternary and Quaternary Nomenclature What is the name of Na. NO 3? A. Sodium nitrite B. Sodium nitride C. Sodium nitrogen trioxide D. Sodium nitrate E. Sodium nitrogen oxide

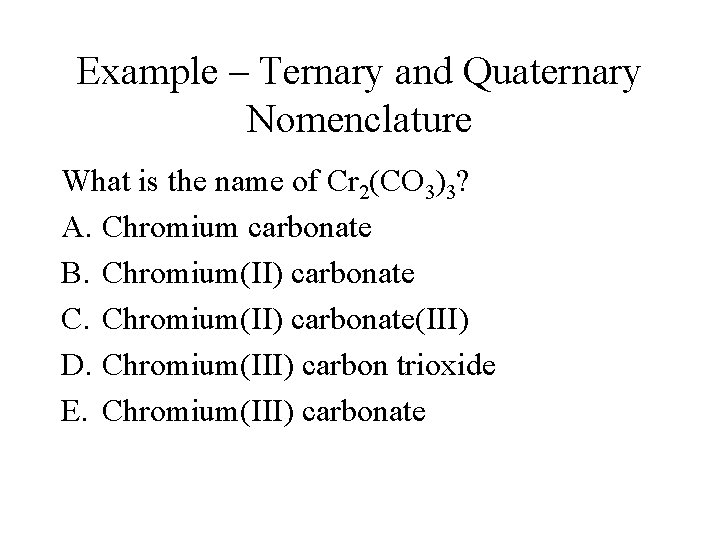
Example – Ternary and Quaternary Nomenclature What is the name of Cr 2(CO 3)3? A. Chromium carbonate B. Chromium(II) carbonate C. Chromium(II) carbonate(III) D. Chromium(III) carbon trioxide E. Chromium(III) carbonate

Example – Acid Nomenclature What is the name of HCl (aq)? A. Chloric acid B. Hydrochloric acid C. Hydrogen chloride D. Hydrochlorous acid E. B and C

Example – Acid Nomenclature What is the name of HCl. O 3 (aq)? A. Chloric acid B. Hydrochloric acid C. Hydrogen chlorate D. chlorous acid E. Perchloric acid

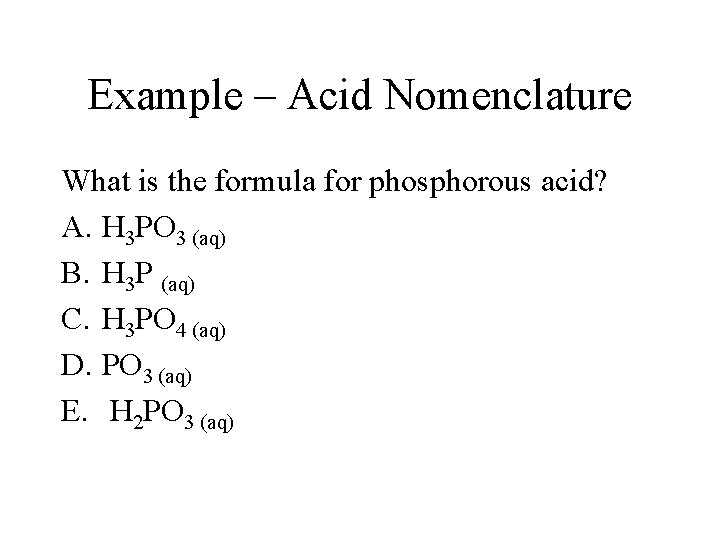
Example – Acid Nomenclature What is the formula for phosphorous acid? A. H 3 PO 3 (aq) B. H 3 P (aq) C. H 3 PO 4 (aq) D. PO 3 (aq) E. H 2 PO 3 (aq)

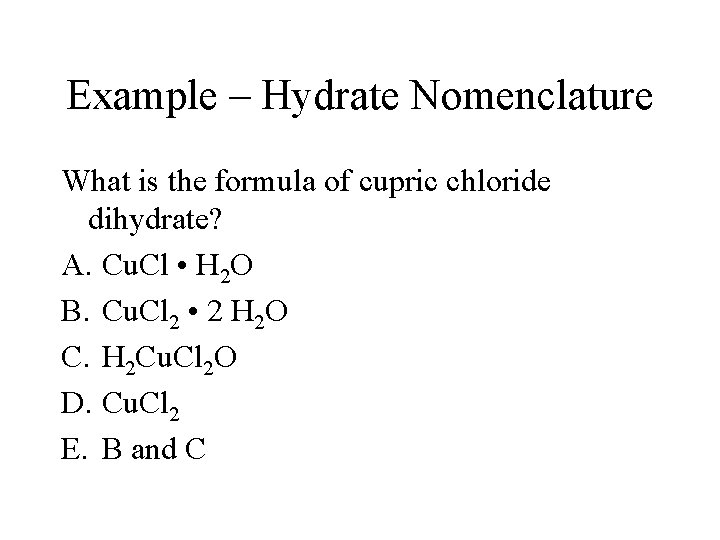
Example – Hydrate Nomenclature What is the formula of cupric chloride dihydrate? A. Cu. Cl • H 2 O B. Cu. Cl 2 • 2 H 2 O C. H 2 Cu. Cl 2 O D. Cu. Cl 2 E. B and C

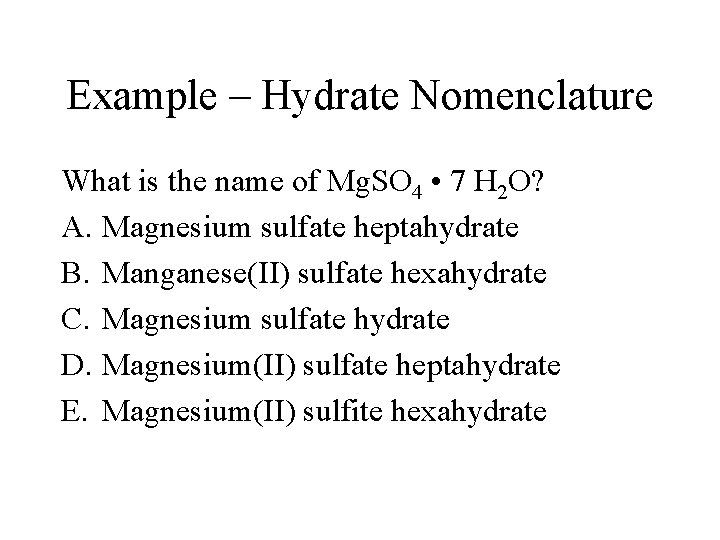
Example – Hydrate Nomenclature What is the name of Mg. SO 4 • 7 H 2 O? A. Magnesium sulfate heptahydrate B. Manganese(II) sulfate hexahydrate C. Magnesium sulfate hydrate D. Magnesium(II) sulfate heptahydrate E. Magnesium(II) sulfite hexahydrate