NOMENCLATURE NOTES Ionic Bonding and Salts Righthand panel

NOMENCLATURE NOTES Ionic Bonding and Salts Right-hand panel of Front side of Notes
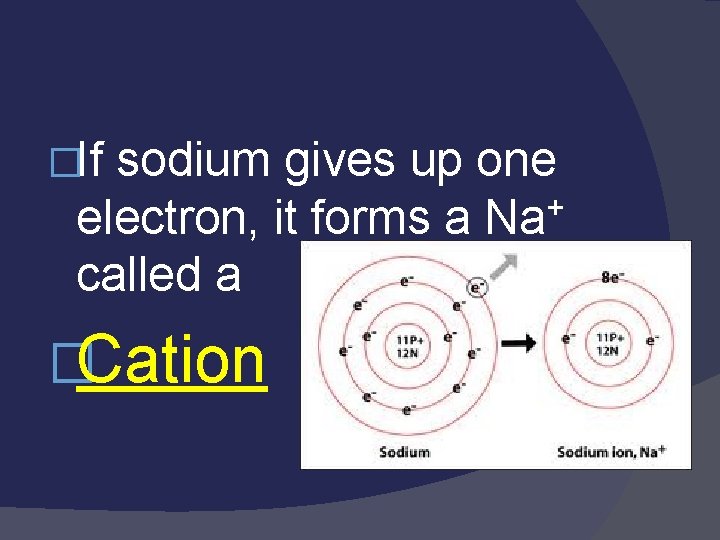
�If sodium gives up one + electron, it forms a Na called a �Cation
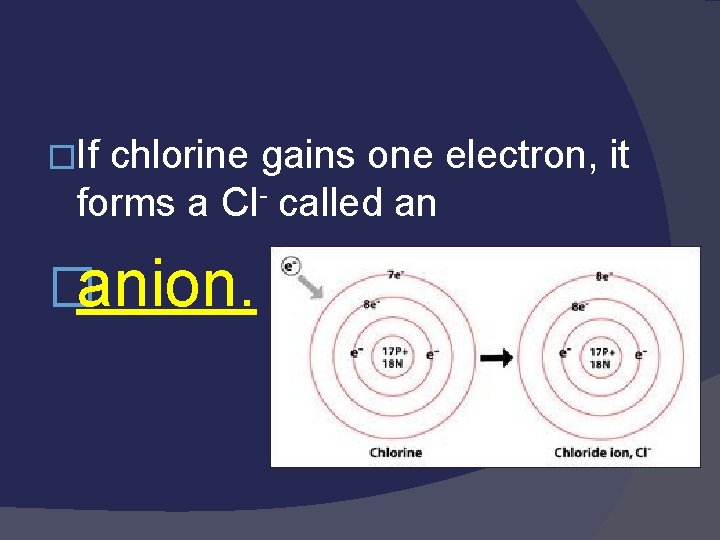
�If chlorine gains one electron, it forms a Cl- called an �anion.

Explain why each of the following pairs is NOT likely to form an ionic bond. �Chlorine and bromine �Both are halogens, anions �Potassium and helium �He is a noble gas �Sodium and lithium �Both are metals, cations

List some of the properties that are characteristic of an ionic compound. �High melting point �High boiling point �Solid at room temperature �Hard and brittle �Nonconductors as solids, but good conductors in the liquid state and when dissolved in water.

When is salt a good conductor of electricity? �Salt melts (molten) �Salt dissolves

� Crystal Lattice The regular pattern of “repeating” units in which a crystal is arranged. energy – energy released when a salt is formed from gaseous ions. �Lattice
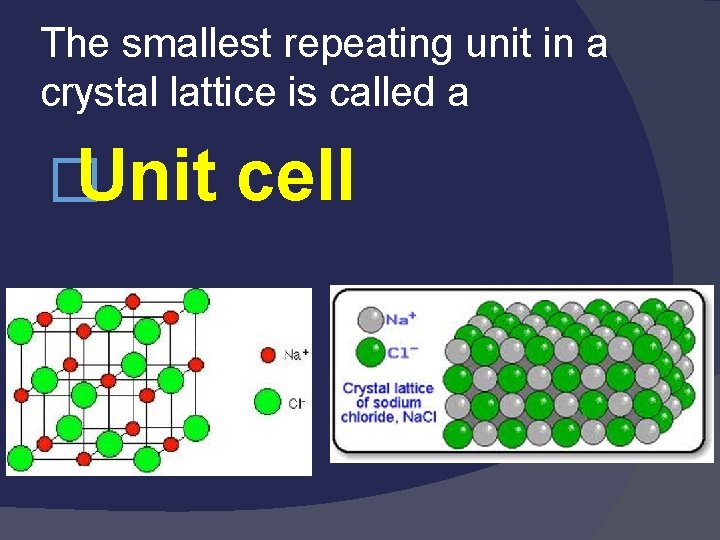
The smallest repeating unit in a crystal lattice is called a �Unit cell
![�The electron configuration for arsenic, As, is [Ar]3 d 104 s 24 p 3. �The electron configuration for arsenic, As, is [Ar]3 d 104 s 24 p 3.](http://slidetodoc.com/presentation_image_h2/5cfd1e108689c2f46d58ca956920adfc/image-9.jpg)
�The electron configuration for arsenic, As, is [Ar]3 d 104 s 24 p 3. How many valence electrons does an As atom have? ○5 �Write the symbol for the ion it forms to achieve a noble gas configuration. -3 ○As

�Explain why magnesium form Mg 2+ cations and not Mg 6 anions. � Takes less energy

�Why can’t an ionic bond form between potassium and magnesium? � Both are metals, cations

�Why can’t sodium gain a positive charge by acquiring a proton in its nucleus? � You would no longer have Na, but another element
- Slides: 12