Nomenclature Naming Neutralizaton Acids Bases the p H

Nomenclature + Naming + Neutralizaton Acids, Bases & the p. H Scale
What are Acids + Bases? �These are created when substances are mixed with water. �Acids: Release hydrogen ions - H+ ions into water. �Bases: Release hydroxide - (OH)ions into water. �BOTH substances are corrosive, meaning they can give you a chemical burn.

Acids �Acids will have the element hydrogen (H) first in their formula. �E. G. HCl or H 2 SO 4 �Examples of acids at home: E. G. Vinegar, Lemon Juice, Coke or Pepsi and batteries. �There are two types of acids: 1. Oxyacids: Have oxygen in their formulas. 2. Non-oxy acids: Don’t have oxygen in their formulas.

Naming Oxyacids: Have oxygen in their formulas. Q: What would you name H 2(SO 4) using the rules you know? � A: Hydrogen Sulphate � Rules for naming this acid: 1. � Drop “hydrogen” from the name “ate” ending “ic acid” “ite” ending “ous acids” � What would the name of this acid H 2(SO 4) be? Sulphuric Acid

Naming Non-oxy acids: Don’t have oxygen in their formulas. � Q: What would you name HCl using the rules you know? � A: Hydrogen Chloride � Rules for naming this acid: 2. Hydrogen “Hydro” “ide” ending “ic acid” � What would the name of this acid HCl be? Hydrochloric Acid

Getting the Formulas for Acids This is the same as before we use the “Xover rule”. � What would the formula be for Hydrophosphoric acid? � First write down the chemical symbols for each element or poly. ion + their charge: � � + H 3 P X-Over the charges to get the formula: H 3 P

Properties of Acids �Sour tasting �Stings to touch �Corrosive �Water soluble �React with metals �Conduct electricity

Uses of Acids �Fruit, drinks (coke, juice), yogurt, vinegar �Batteries �Catalyst �Sore muscles

Bases �Bases will have the hydroxide (OH-) group second in their formula. �E. G. K(OH) or Na(OH) �Examples of bases at home: E. G. Bleach, Dishsoap, Antacid tablets, Gaviscon or Draincleaner
Bases – Formulas + Naming �The rules for naming bases is the same as before: The metal name stays the same and so does the name of the polyatomic ion (hydroxide). �What would KOH and Na. OH be called? Potassium Hydroxide Sodium Hydroxide �To get the formulas we use the “X-Over rule”. �What would the formula be for Lithium Hydroxide? Li(OH)

Properties of Bases �Bitter to taste �Slippery to touch �Corrosive �Water soluble �Reactive �Conductive

Safety �Both corrosive �Acids must be added to water for diluting �Bases react with protein – can cause blindness!
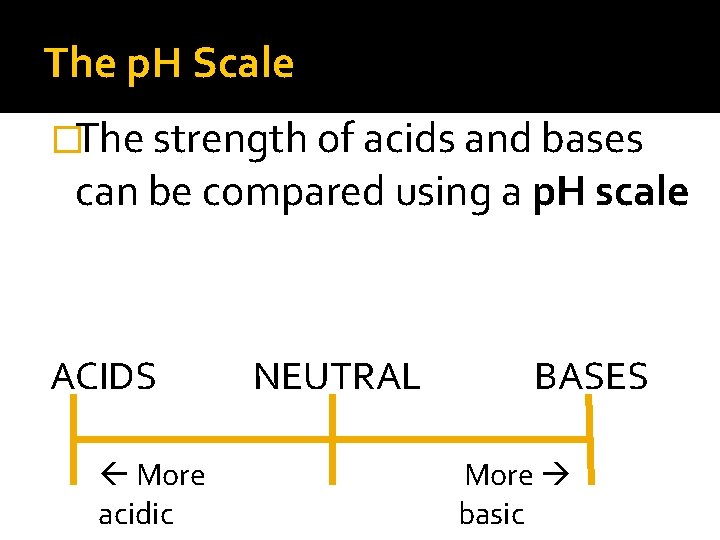
The p. H Scale �The strength of acids and bases can be compared using a p. H scale ACIDS More acidic NEUTRAL BASES More basic
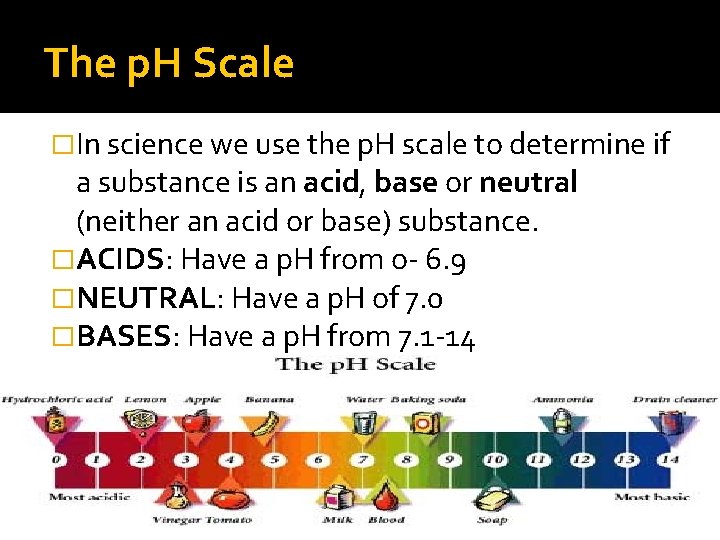
The p. H Scale �In science we use the p. H scale to determine if a substance is an acid, base or neutral (neither an acid or base) substance. �ACIDS: Have a p. H from o- 6. 9 �NEUTRAL: Have a p. H of 7. 0 �BASES: Have a p. H from 7. 1 -14
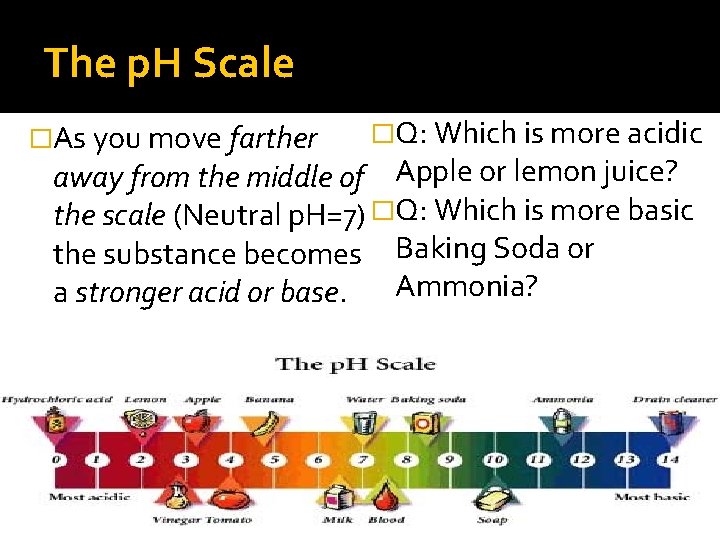
The p. H Scale �As you move farther �Q: Which is more acidic away from the middle of Apple or lemon juice? the scale (Neutral p. H=7) �Q: Which is more basic the substance becomes Baking Soda or a stronger acid or base. Ammonia?
Indicators: Is it an Acid or Base? �Q: What if the p. H or formula isn’t given to you, how can you tell if it’s an acid, neutral or base? �A: By using indicators. �Indicators are substances that change colours when they contact an acid, base or neutral. �We can observe the colour change of the indicator to determine if it’s an acid, base or neutral.
DEMOs �Pop Rocks + Water with Bromothymol blue indicator. �Water + Breath with Bromothymol Blue �Comparing Indicators in Acid, Base + Neutral Substances
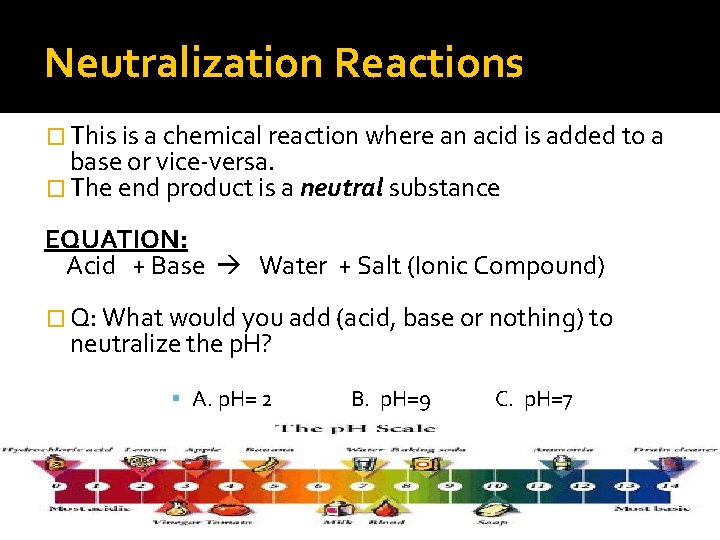
Neutralization Reactions � This is a chemical reaction where an acid is added to a base or vice-versa. � The end product is a neutral substance EQUATION: Acid + Base Water + Salt (Ionic Compound) � Q: What would you add (acid, base or nothing) to neutralize the p. H? A. p. H= 2 B. p. H=9 C. p. H=7
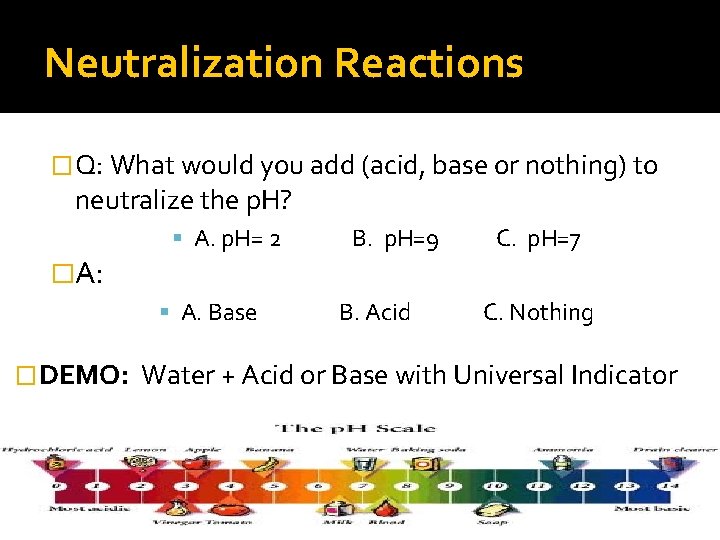
Neutralization Reactions � Q: What would you add (acid, base or nothing) to neutralize the p. H? �A: A. p. H= 2 A. Base � DEMO: B. p. H=9 B. Acid C. p. H=7 C. Nothing Water + Acid or Base with Universal Indicator
- Slides: 19