Nomenclature Naming Compounds Naming Binary Ionic Compounds Name

Nomenclature Naming Compounds

Naming Binary Ionic Compounds

• Name positive element first with its normal name • Name negative element last & change its ending to -ide
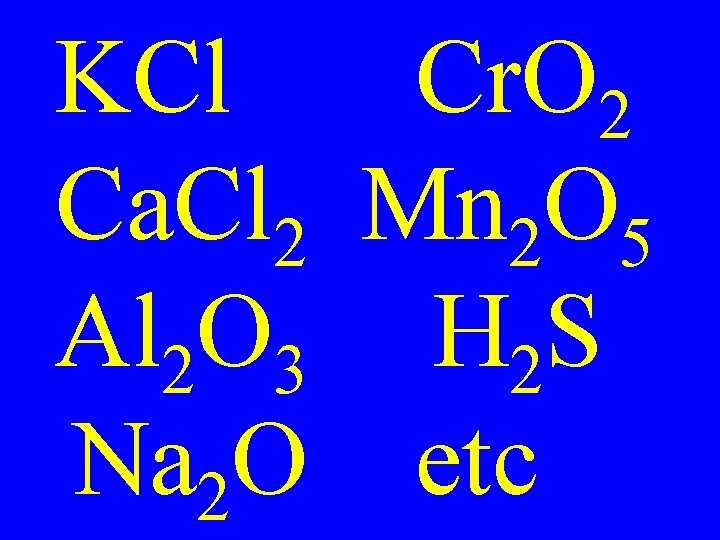
KCl Cr. O 2 Ca. Cl 2 Mn 2 O 5 Al 2 O 3 H 2 S Na 2 O etc

Name Each: Fe. Cl 2 Fe. Cl 3

If the Positive element is not from columns I or II • its ox # must be determined and written in roman numerals

Determining the Charge 1) Add up the oxidation numbers of all the negative elements 2) The positive portion must balance out the negative portion 3) Divide the positive portion by the metal subscript
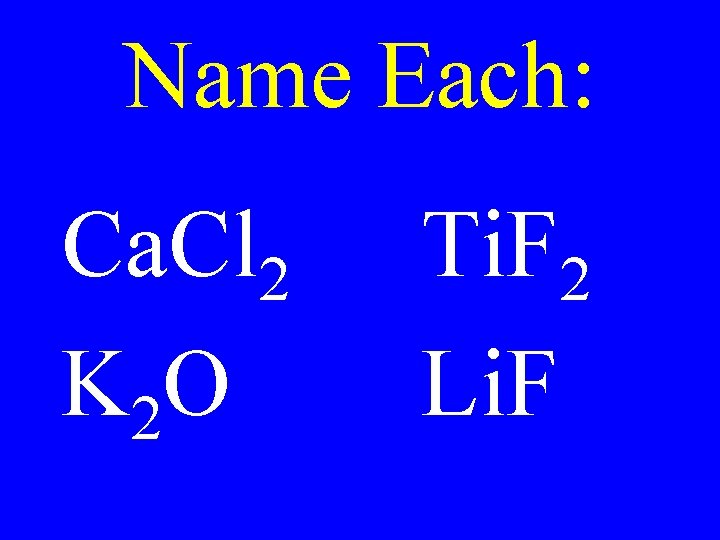
Name Each: Ca. Cl 2 K 2 O Ti. F 2 Li. F

Deriving Formulas • Write the symbol for each element • Determine each element’s charge & apply subscripts to balance charge

Derive Formulas • Magnesium chloride • Lead(II)sulfide • Iron(III)carbide

• Same rules as ionic compounds except: • use geometric prefixes to determine the # of each atom
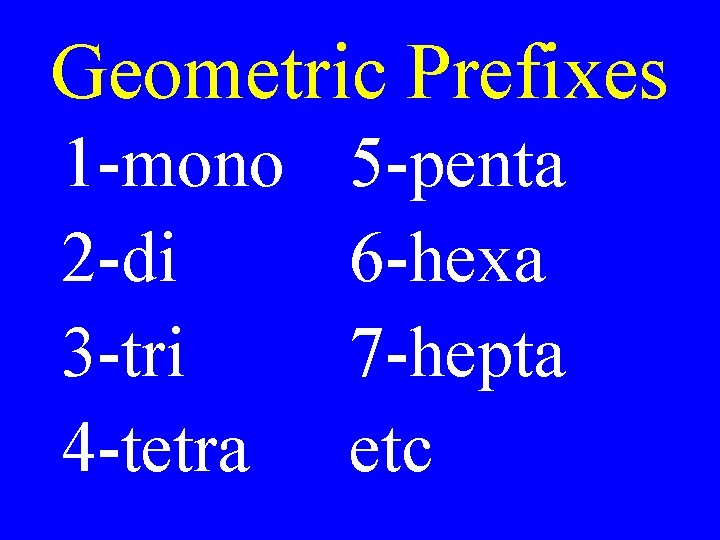
Geometric Prefixes 1 -mono 2 -di 3 -tri 4 -tetra 5 -penta 6 -hexa 7 -hepta etc
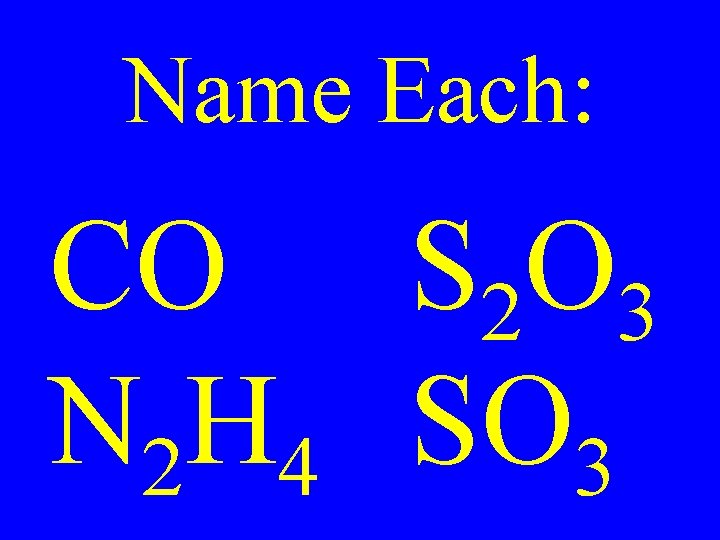
Name Each: CO S 2 O 3 N 2 H 4 SO 3

Write formulas for: • Sodium sulfide • Lead (II) iodide • Diphosphorus pentoxide

Write formulas for: • Chromium(III) oxide • Aluminum carbide • Dibromine heptoxide

Polyatomic Ion

• A group of atoms chemically combined that together has a charge

• Most are oxoanions -3 -2 • PO 4 SO 4 • A root element bound to oxygen

Naming Polyatomic Ions

Google Polyatomic Ion Chart at Home & Learn the Chart

• Learn the polyatomic table on the internet • Learn how to use the periodic table to determine polyatomic ions

• Name the root element • Change the ending to -ate -3 • PO 4 = phosphate • Some are unusual
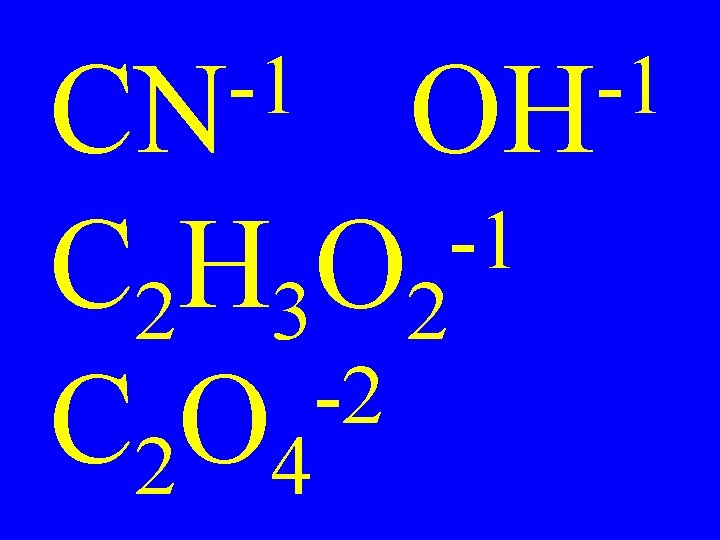
-1 CN -1 OH C 2 H 3 O 2 -2 C 2 O 4 -1
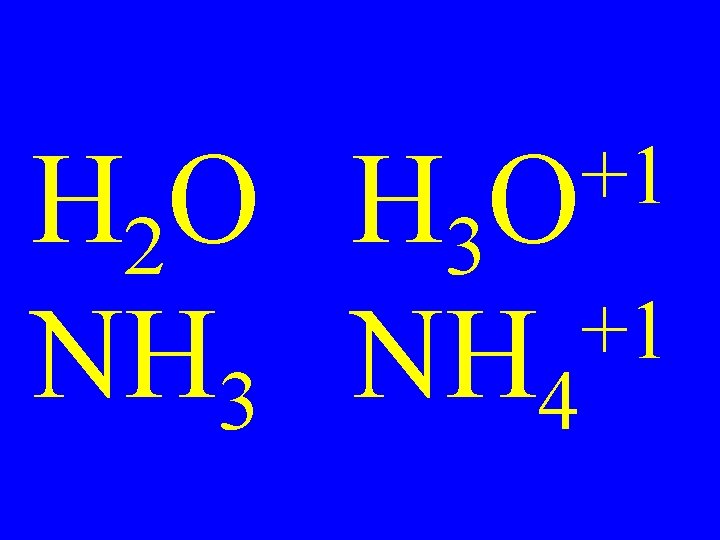
+1 O H 2 O H 3 +1 NH 3 NH 4
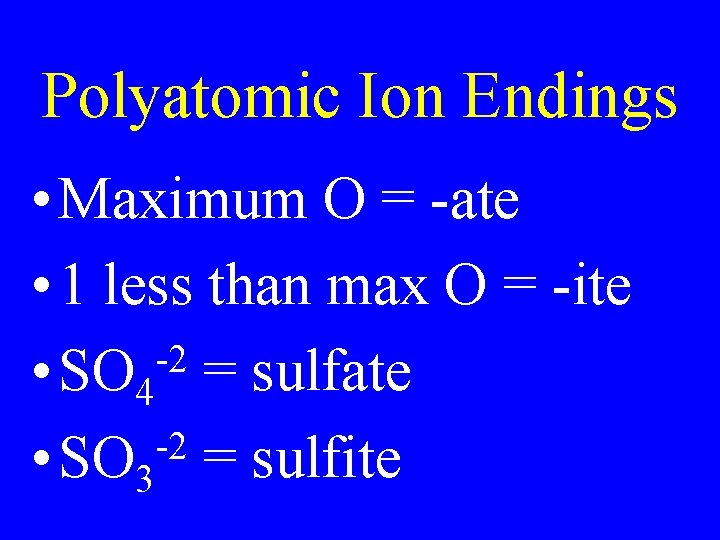
Polyatomic Ion Endings • Maximum O = -ate • 1 less than max O = -ite -2 • SO 4 = sulfate -2 • SO 3 = sulfite

Naming Ternary Compounds

• Follow ionic rules for naming the compound • Name the polyatomic ion as the positive or negative portion

Name Each: Ca. CO 3 K 2 SO 4
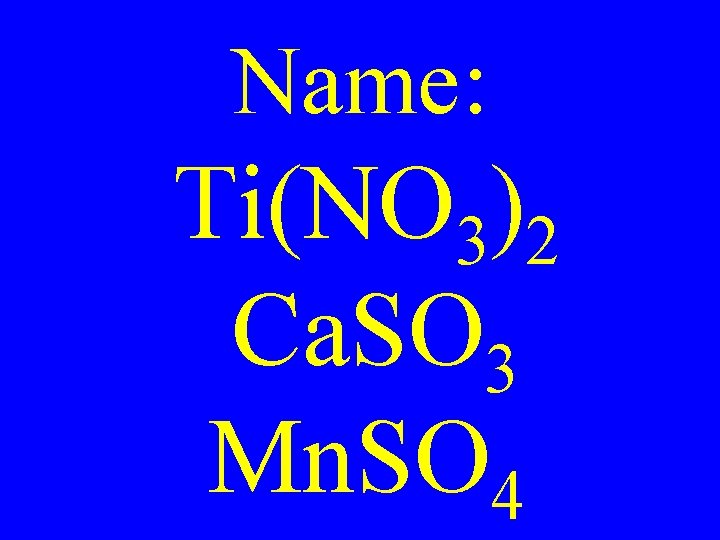
Name: Ti(NO 3)2 Ca. SO 3 Mn. SO 4

Write Formulas For: • Lead (II) nitrate • Aluminum sulfate • Potassium chromate • Ammonium phosphite
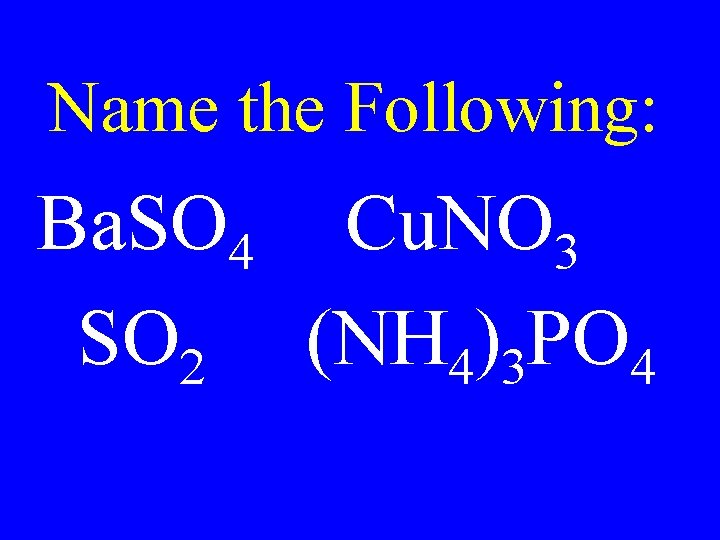
Name the Following: Ba. SO 4 Cu. NO 3 SO 2 (NH 4)3 PO 4

Column 7 Dilemma for Polyatomic Ions • There are 2 polyatomic names (ate & -ite) for each root element. • Polyatomic ions from column 7 have 4 possibilities
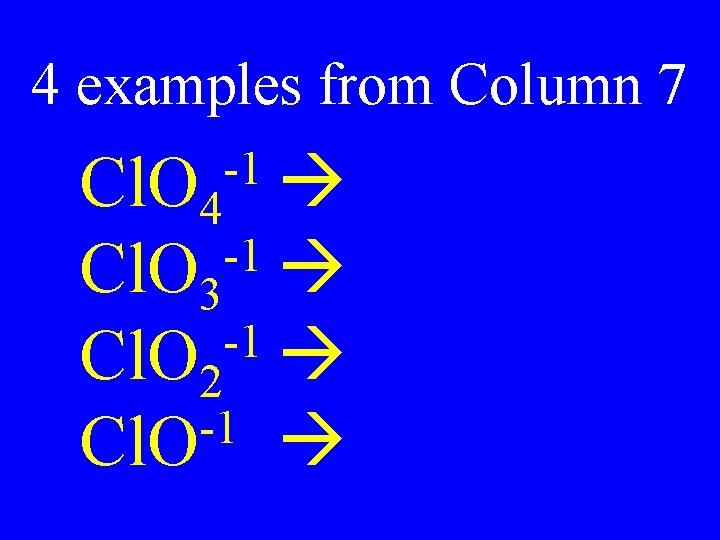
4 examples from Column 7 -1 Cl. O 4 -1 Cl. O 3 -1 Cl. O 2 -1 Cl. O
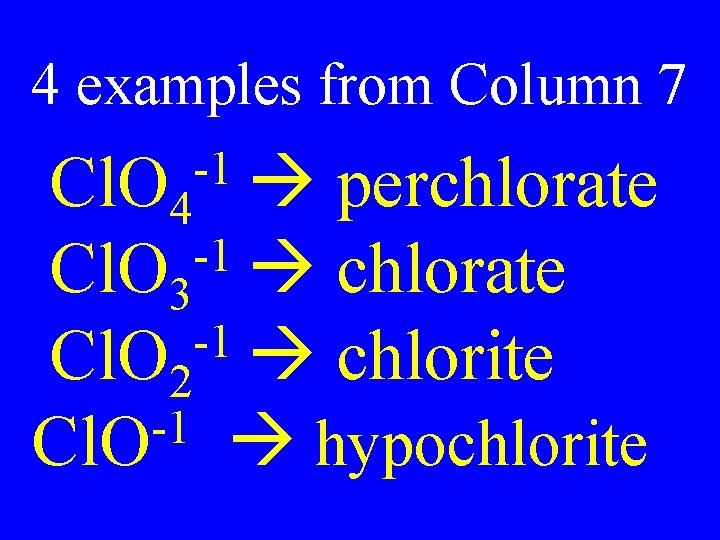
4 examples from Column 7 -1 Cl. O 4 perchlorate -1 Cl. O 3 chlorate -1 Cl. O 2 chlorite -1 Cl. O hypochlorite

Also true for others IO 4 periodate -1 IO 3 iodate -1 IO 2 iodite -1 IO hypoiodite -1

Naming Acids

• ___ ide ions become: • hydro ___ ic acids • ___ ate ions become: • ___ ic acids • ___ ite ions become: • ___ ous acids

Name or Give Formulas For: • HBr(aq) H 2 SO 4(aq) • Na. NO 3 (NH 4)3 PO 3 • Phosphoric acid • Nitric acid • Chloric acid

Derive formulas for each: • Cesium oxide • Barium chloride • Calcium phosphide • Aluminum sulfide

Write Formulas For: • Magnesium nitrate • Aluminum phosphate • Potassium nitrite • Ammonium chloride
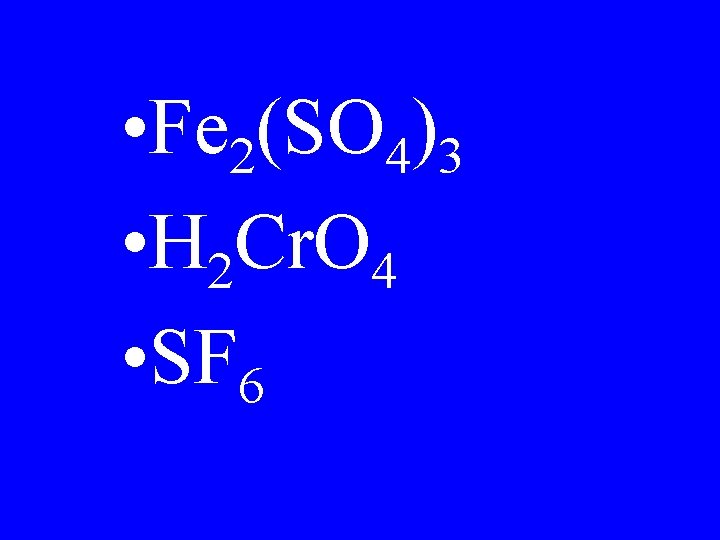
• Fe 2(SO 4)3 • H 2 Cr. O 4 • SF 6

Review for Test 1

Classify types of matter • Dirt • Sodium • Glucose • Vodka

Classify properties • Color • Shape • Ability to burn

Convert the following: • 2. 1 mg to mg • 3. 2 km to cm • 4. 8 Gm/Ms to mm/ns
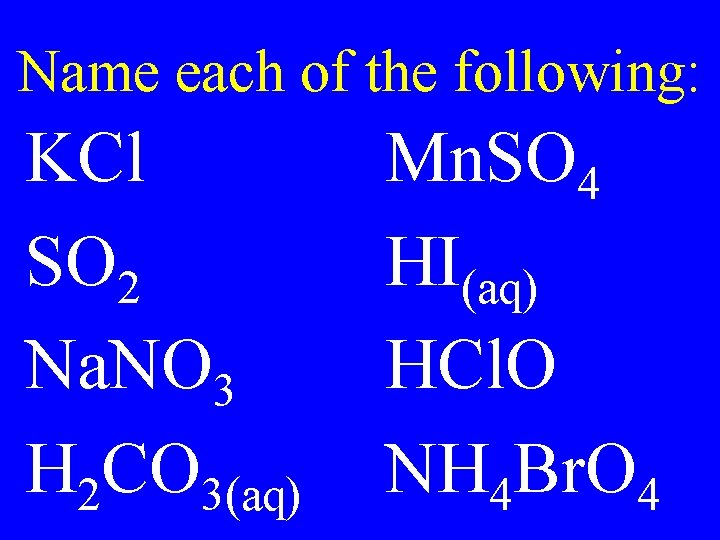
Name each of the following: KCl SO 2 Na. NO 3 H 2 CO 3(aq) Mn. SO 4 HI(aq) HCl. O NH 4 Br. O 4
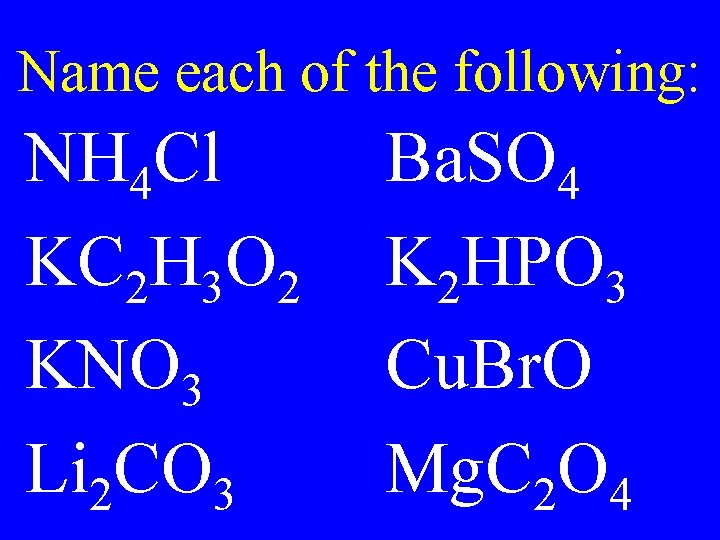
Name each of the following: NH 4 Cl KC 2 H 3 O 2 KNO 3 Li 2 CO 3 Ba. SO 4 K 2 HPO 3 Cu. Br. O Mg. C 2 O 4

Derive formulas for each: • Silicon dioxide • phosphorus trichloride • Sulfur hexafluoride • Iodine trifluoride

Derive formulas for each: • Potassium sulfate • Lead(II)chromate • Aluminum hydroxide • Ammonium cyanide
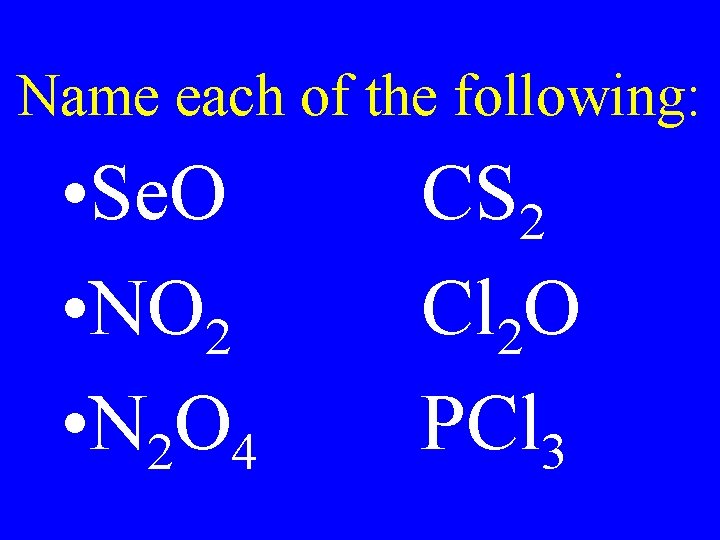
Name each of the following: • Se. O • NO 2 • N 2 O 4 CS 2 Cl 2 O PCl 3
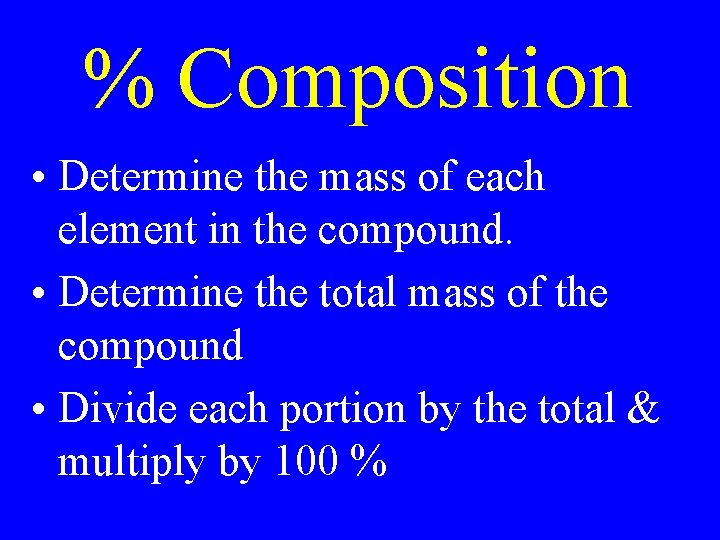
% Composition • Determine the mass of each element in the compound. • Determine the total mass of the compound • Divide each portion by the total & multiply by 100 %
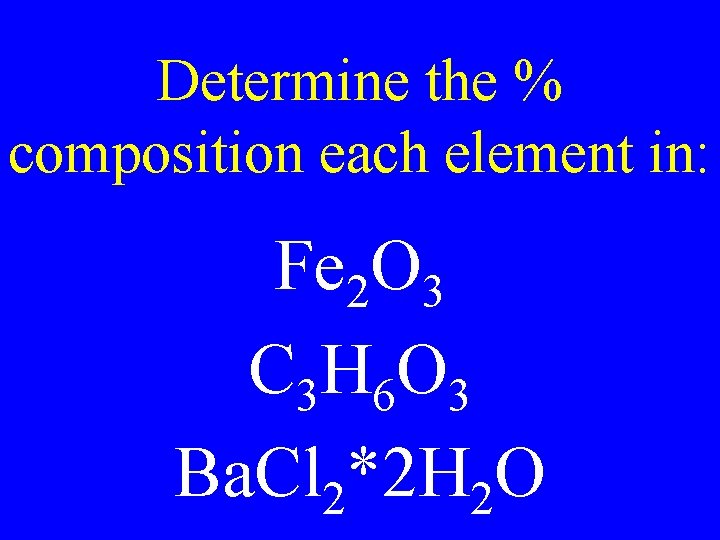
Determine the % composition each element in: Fe 2 O 3 C 3 H 6 O 3 Ba. Cl 2*2 H 2 O

Name each of the following: -2 • SO 4 -3 • PO 4 -1 • Cl. O 2 -2 SO 3 -1 NO 3 -1 Cl. O

Derive formulas for each: Chromate Arsenite Bromite Sulfate Nitrate Cyanide Acetate
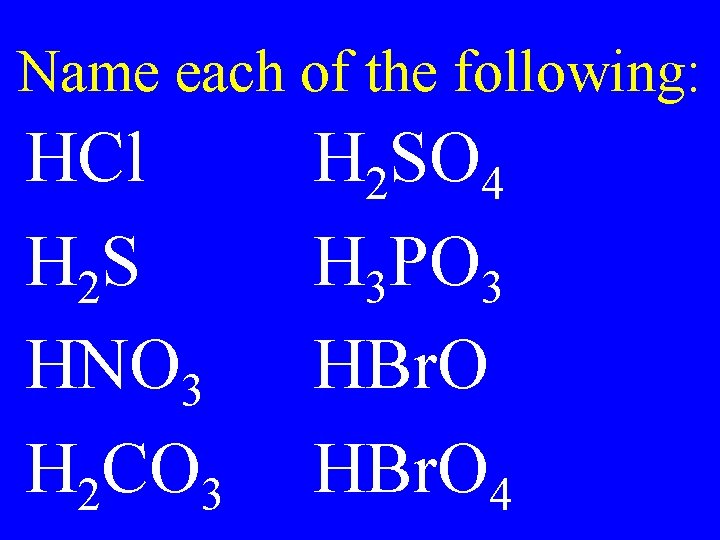
Name each of the following: HCl H 2 S HNO 3 H 2 CO 3 H 2 SO 4 H 3 PO 3 HBr. O 4

Derive formulas for each: • Chromic acid • Hydroiodic acid • Sulfurous acid • Bromic acid
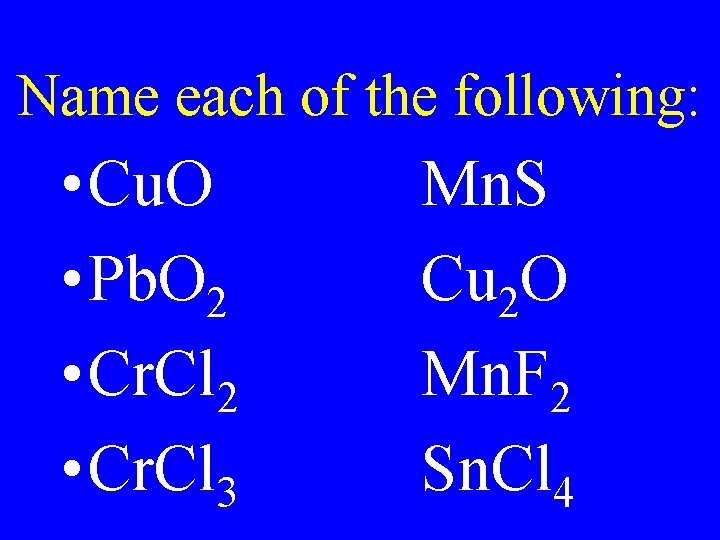
Name each of the following: • Cu. O • Pb. O 2 • Cr. Cl 3 Mn. S Cu 2 O Mn. F 2 Sn. Cl 4

Name each of the following: Fe. O Fe 2 O 3

Derive formulas for each: • Lead(IV)oxide • Copper(II)sulfide • Manganese(VII)oxide • Nickel(II)fluoride
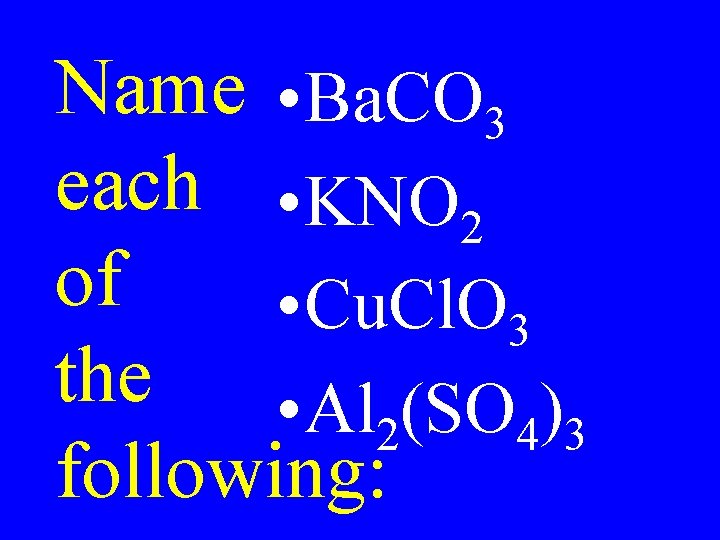
Name • Ba. CO 3 each • KNO 2 of • Cu. Cl. O 3 the • Al 2(SO 4)3 following:
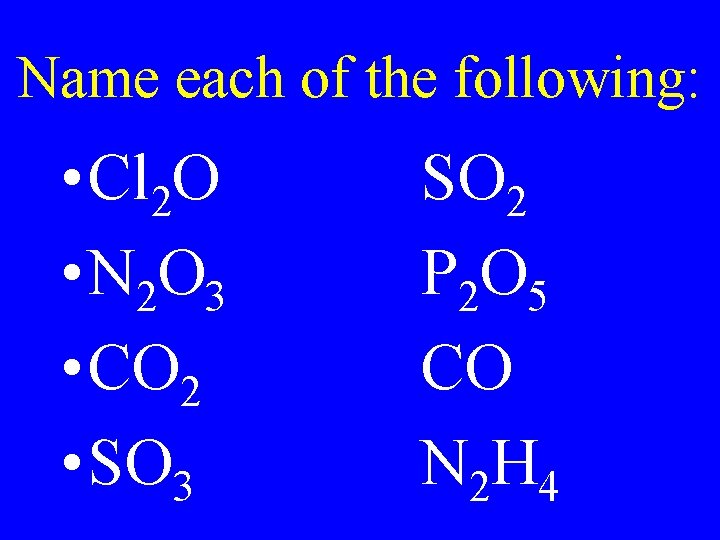
Name each of the following: • Cl 2 O • N 2 O 3 • CO 2 • SO 3 SO 2 P 2 O 5 CO N 2 H 4
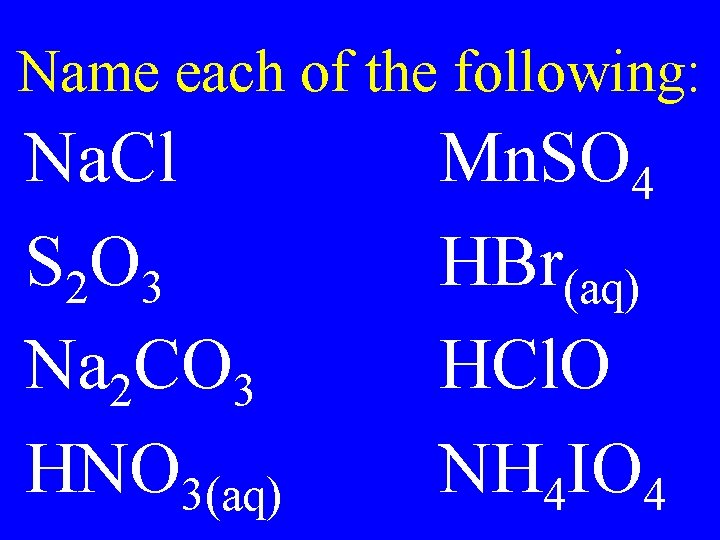
Name each of the following: Na. Cl S 2 O 3 Na 2 CO 3 HNO 3(aq) Mn. SO 4 HBr(aq) HCl. O NH 4 IO 4
- Slides: 62