Nomenclature II Alkynes Alkenes and Benzene rings Simple

Nomenclature II Alkynes, Alkenes, and Benzene rings
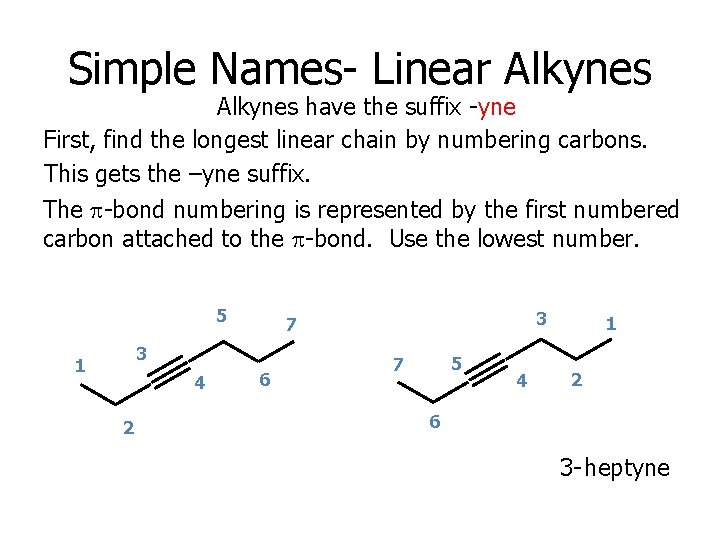
Simple Names- Linear Alkynes have the suffix -yne First, find the longest linear chain by numbering carbons. This gets the –yne suffix. The p-bond numbering is represented by the first numbered carbon attached to the p-bond. Use the lowest number. 5 3 1 4 2 3 7 6 5 7 4 1 2 6 3 - heptyne
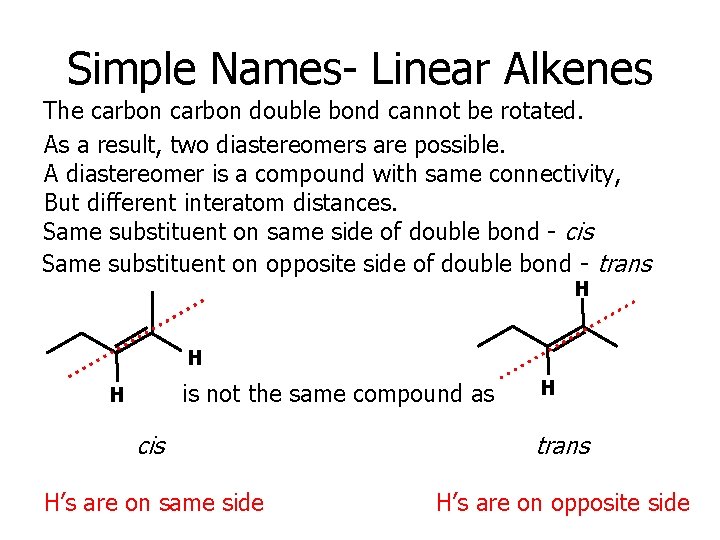
Simple Names- Linear Alkenes The carbon double bond cannot be rotated. As a result, two diastereomers are possible. A diastereomer is a compound with same connectivity, But different interatom distances. Same substituent on same side of double bond - cis Same substituent on opposite side of double bond - trans H H is not the same compound as H H cis trans H’s are on same side H’s are on opposite side

Simple Names- Linear Alkenes have the suffix -ene First, find the longest linear chain by numbering carbons. This gets the –ene suffix. The p-bond numbering is represented by the first numbered carbon attached to the p-bond. Use the lowest number. The cis/trans label is placed in front of the name to distinguish between the two diastereomers. 2 1 4 3 5 8 8 6 7 cis-2 -nonene 9 9 6 7 2 4 5 3 1 trans- 2 - nonene

Simplest Alkene: Ethylene 2 C’s Ethene/ethylene suffix or vinyl prefix. F Br bromoethylene or vinylbromide F 1, 1 -difluoroethylene Br Cl cis 2 -bromo-1 -chloroethylene or cis 1 -bromo-2 -chloroethylene

Tri and Tetrasubstituted Alkenes The cis and trans prefix really should only be used with disubstituted alkenes. When three or four nonhydrogen substituents are attached to an alkene, the E or Z system should be used. E is short for entgegen (German for opposite). Z is short for zusammen (German for together). H Z is for Zee Zame Zide H H cis-2 -pentene or Z-2 -pentene H trans-2 -pentene or E-2 -pentene

Assigning E/Z Divide the molecule in half, by cutting perpendicular to the C=C bond. Assign priority 1 or 2 on each side of the C=C bond by atomic number of the atom directly attached to the alkene. If there is a tie, then list the atoms directly attached to the atom attached to the alkene. Find the atom with the highest atomic number in each set. If there still is a tie, look at the next highest number. Keep repeating the process until there is a difference. Draw a dashed line along the alkene. If the 1’s are on the Zee Zame Zide, then it is a Z alkene, otherwise it is an E alkene. CHH 6>1 H 2 1 1 6>1 6=6 2 HHH E E-3 -methyl-2 -pentene

Exam Level Questions: Assign E/Z to the following alkenes. Some molecules have more than one alkene that need E/Z assignment. Some alkenes cannot be assigned. You do not need to name the molecule itself. 1. 2. Z 3. Z 4. E E Z 5. CCH E CCH Z CHH 6. 7. E E CCC (Carbon carbon double bonds count as two bonds to carbon) E

Benzene is a special type of molecule called an aromatic ring. Even though it contains p bonds, it really is not an alkene as it is immune to many reactions that alkenes are susceptible. There are some special names that relate to the benzene ring.

Naming Benzene Rings There are two ways to name a benzene ring: Benzene (if it is main part of molecule) or phenyl prefix if it is a side chain. bromobenzene or phenylbromide (Ph. Br) fluorobenzene chlorobenzene or or phenylchloride (Ph. Cl) phenylfluoride (Ph. F) iodobenzene or phenyliodide (Ph. I) Benzyl prefix: A phenyl group attached to a CH 2 benzyl bromide benzyl chloride benzyl fluoride benzyl iodide

Exam Level Questions: Draw the following: trans-3 -heptene Z-3 -heptene E-3 -methyl-4 -isopropyl-2 -octene 1 -benzyl-1 -bromoethylene E-2 -phenyl-2 -butene triphenylmethane
- Slides: 11