Nomenclature Honors Coordinated Science II WheatleyHeckman Ionic Compounds







- Slides: 18

Nomenclature Honors Coordinated Science II Wheatley-Heckman

Ionic Compounds • Held together by ionic bonds. • What are ionic bonds? – Between metals and nonmetals – Transfer of electrons between atoms. – Attraction between oppositely charged ions.

Ionic Compounds • Some examples are: – – Na. Cl Mg. Cl 2 KBr Li. O 2 • These are all bonds between cations (positive ions) and anions (negative ions).

Naming Ionic Compounds • Binary Ionic Compounds – The metal will be your cation. Its name comes first. – The non-metal will be your anion. Its name comes second, but undergoes a slight change. • Change the ending to –ide. • Example: Chlorine Chloride, Nitrogen Nitride • Li. O 2 Lithium + Oxygen = Lithium Oxide

Practice • Name the following ionic compounds: – – – Na. Cl Rb 2 S Be. F 2 Cs 3 N KI – In ionic compounds, the subscripts do NOT affect the name of the compound.

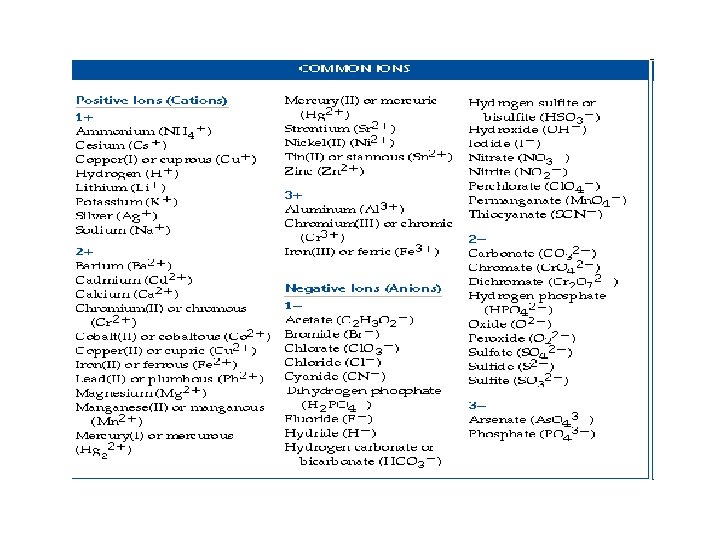
Naming Ionic Compounds • Polyatomic Ions – Ions made of multiple atoms. – Name using an ion chart, but otherwise name the same as binary compounds. – Examples: – Mg. CO 3 = Magnesium Carbonate – (NH 4)SO 4 = Ammonium Sulfate


Writing Formulas from Names • The key to writing correct ionic formulas is to balance your positive and negative charges. We want a neutral molecule. • The charge of the cation becomes the subscript of the anion. • The charge of the anion becomes the subscript of the cation.

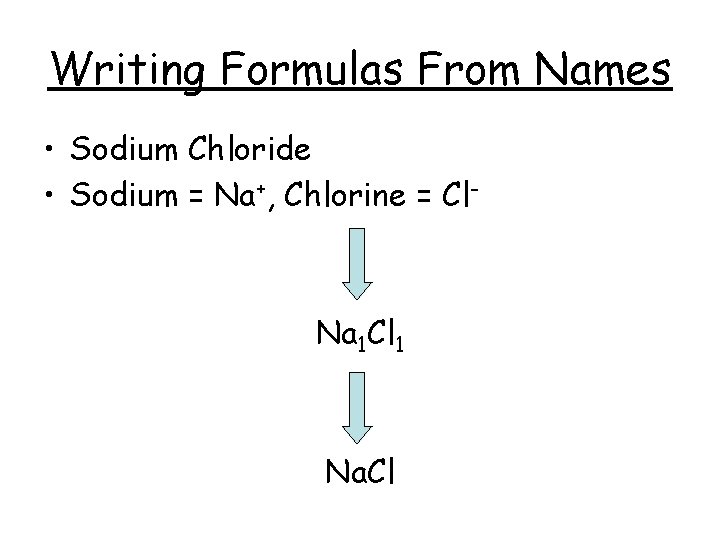
Writing Formulas From Names • Sodium Chloride • Sodium = Na+, Chlorine = Cl- Na 1 Cl 1 Na. Cl

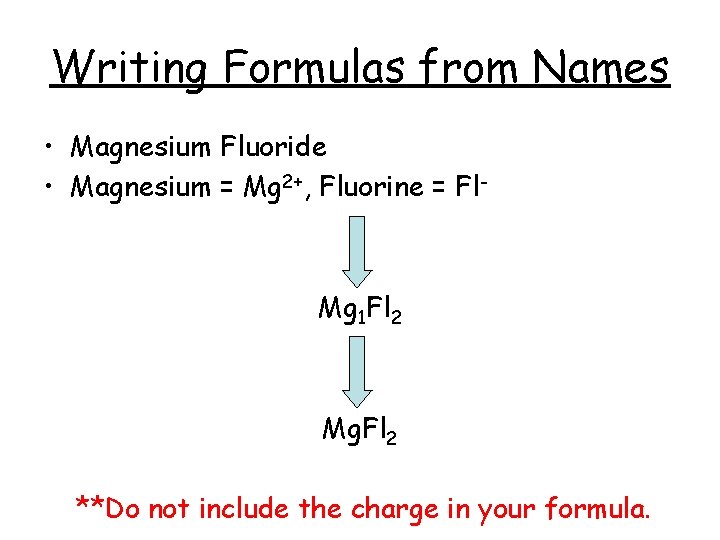
Writing Formulas from Names • Magnesium Fluoride • Magnesium = Mg 2+, Fluorine = Fl- Mg 1 Fl 2 Mg. Fl 2 **Do not include the charge in your formula.

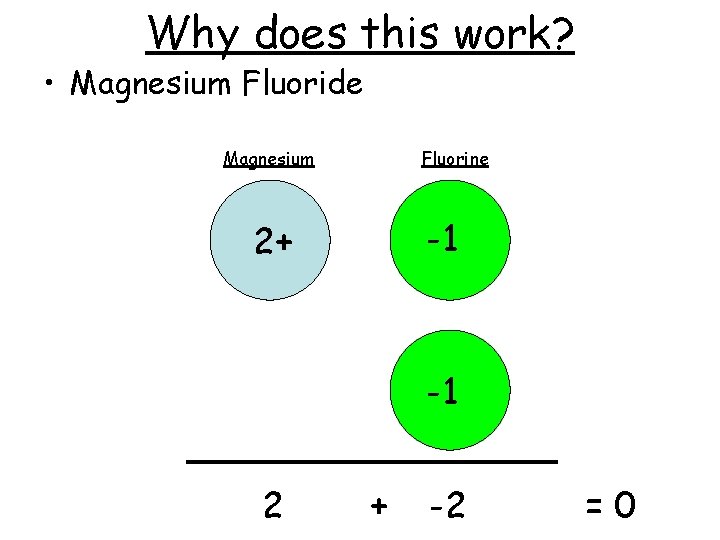
Why does this work? • Magnesium Fluoride Magnesium Fluorine -1 2+ -1 2 + -2 =0

Ionic Naming using Transition Metals • The charges of transition metals can vary. • A metal can have different charges depending on the situation. • You can use the formula to determine its charge in a specific molecule.

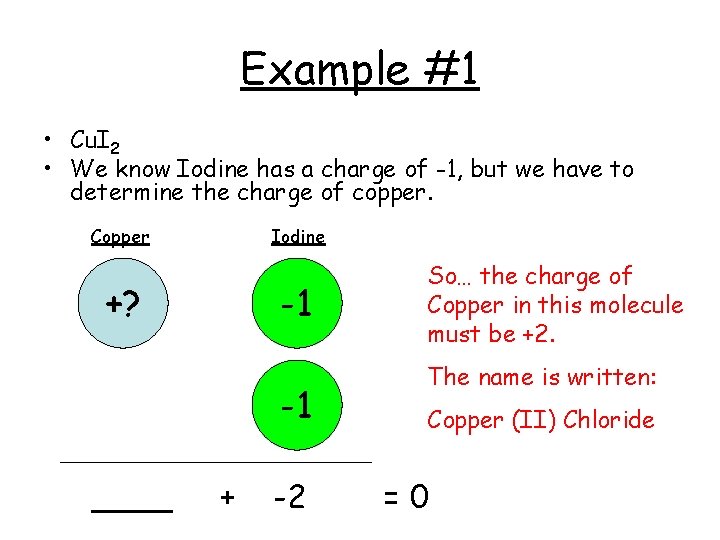
Example #1 • Cu. I 2 • We know Iodine has a charge of -1, but we have to determine the charge of copper. Copper Iodine +? -1 -1 ____ + -2 So… the charge of Copper in this molecule must be +2. The name is written: Copper (II) Chloride =0

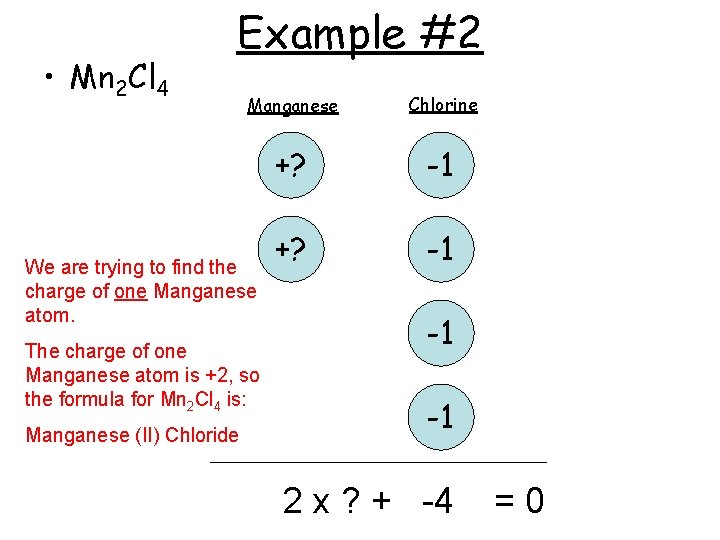
• Mn 2 Cl 4 Example #2 Manganese Chlorine +? -1 We are trying to find the charge of one Manganese atom. The charge of one Manganese atom is +2, so the formula for Mn 2 Cl 4 is: Manganese (II) Chloride -1 -1 2 x ? + -4 =0

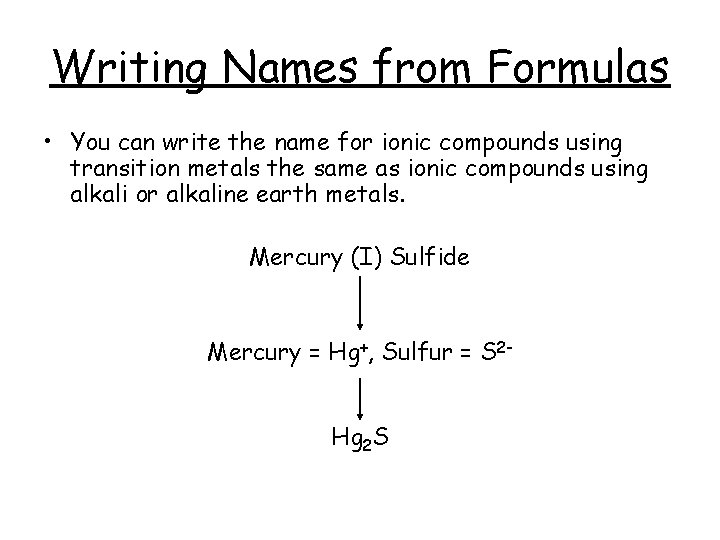
Writing Names from Formulas • You can write the name for ionic compounds using transition metals the same as ionic compounds using alkali or alkaline earth metals. Mercury (I) Sulfide Mercury = Hg+, Sulfur = S 2 Hg 2 S

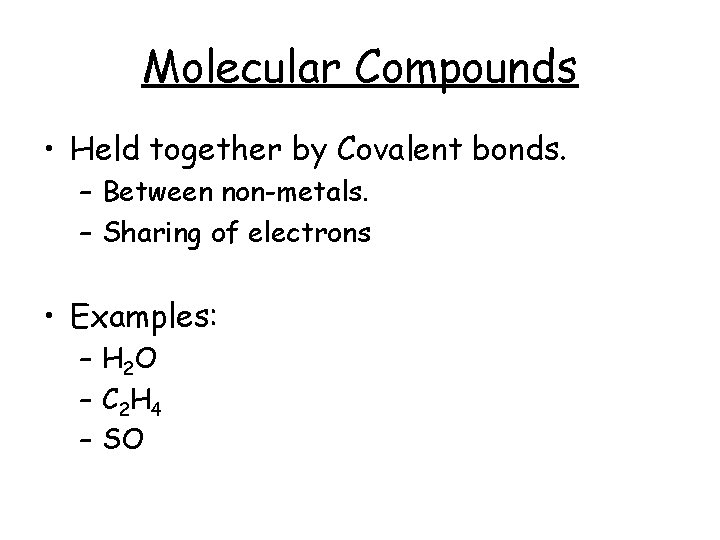
Molecular Compounds • Held together by Covalent bonds. – Between non-metals. – Sharing of electrons • Examples: – H 2 O – C 2 H 4 – SO

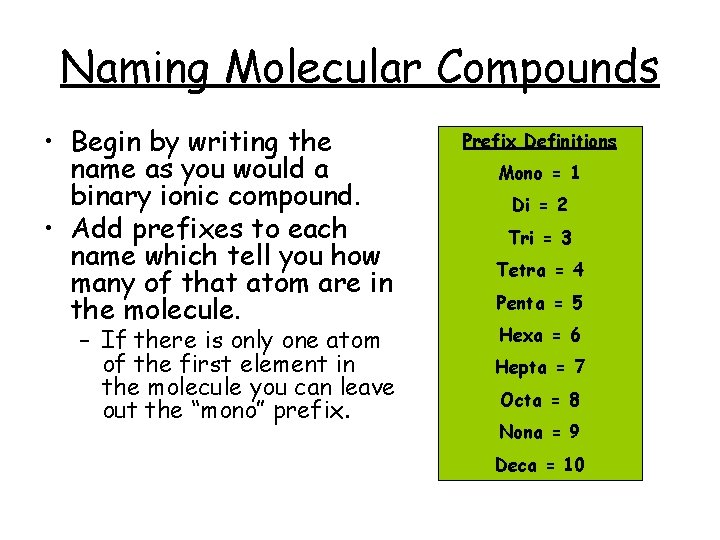
Naming Molecular Compounds • Begin by writing the name as you would a binary ionic compound. • Add prefixes to each name which tell you how many of that atom are in the molecule. – If there is only one atom of the first element in the molecule you can leave out the “mono” prefix. Prefix Definitions Mono = 1 Di = 2 Tri = 3 Tetra = 4 Penta = 5 Hexa = 6 Hepta = 7 Octa = 8 Nona = 9 Deca = 10

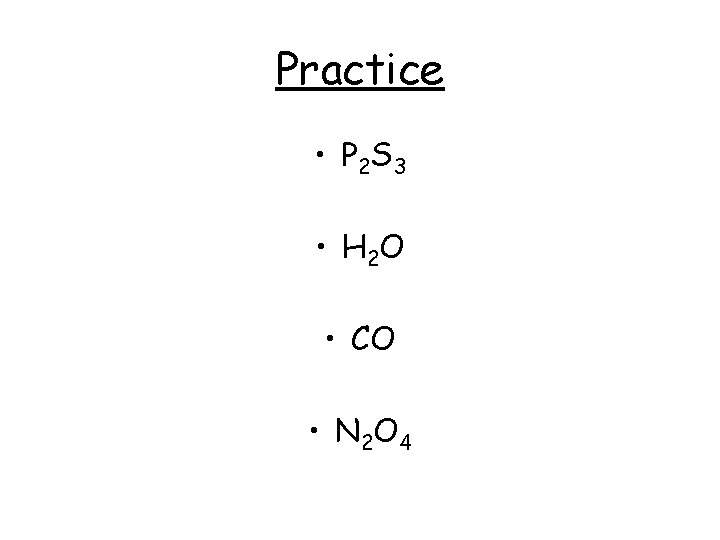
Practice • P 2 S 3 • H 2 O • CO • N 2 O 4