Nomenclature Fancy word for naming and writing formulas


























- Slides: 34

Nomenclature Fancy word for naming and writing formulas

Video https: //www. youtube. com/watch? v= z. GM-w. SKFBpo

Video https: //www. youtube. com/watch? v= Vg. VQKCcfwn. U

Where do the element symbols come from? • Most come from the first letter or the first two letters of their full name – C, O, Al, Ar, Be, Ba etc • Some from the first letter and another letter in their name – Cd, Cr, Cl, Mg • Some from their Latin names – Sb, Au, Fe, Pb • But for all of them, the first letter is always capitalized and the second is always lower case.

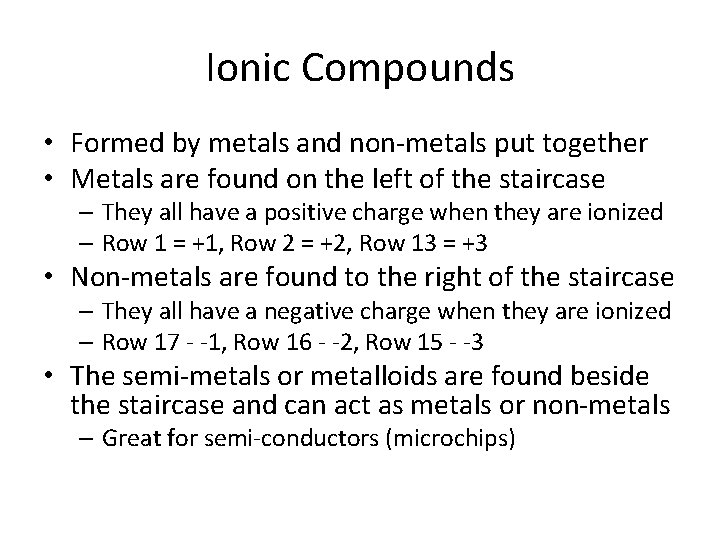
Ionic Compounds • Formed by metals and non-metals put together • Metals are found on the left of the staircase – They all have a positive charge when they are ionized – Row 1 = +1, Row 2 = +2, Row 13 = +3 • Non-metals are found to the right of the staircase – They all have a negative charge when they are ionized – Row 17 - -1, Row 16 - -2, Row 15 - -3 • The semi-metals or metalloids are found beside the staircase and can act as metals or non-metals – Great for semi-conductors (microchips)

Some terms to know • Cation – Positively charged ion (metals when they lose electrons) • Anion – Negatively charged ion (non-metals when they gain electrons) • Monatomic – Made up of one atom – Ne, H, O, Cl-, H+ • Diatomic – Made up of two atoms – O 2, NO, CO • Triatomic – Made up of three atoms – NO 2, CO 2, H 2 O • Polyatomic – Made up of many atoms – O 2, NO 2, H 3 PO 4

Rules for writing chemical formulas of non-multivalent ionic compounds • Metals are always written first, non-metals second • Write the charge of each ion as superscripts found from the periodic table • Balance the charges by finding the LCM of the charges • Use subscripts to show many of each ion you need to make it balance • Reduce if you can • The final product should be neutral. Positive charge should equal negative charge. You can remove the superscripts. • Remove any 1’s as it is assumed in the final formula

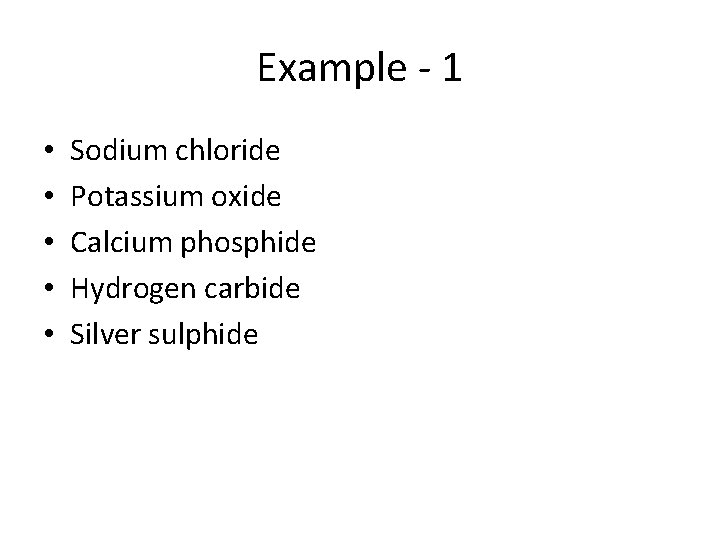
Example - 1 • • • Sodium chloride Potassium oxide Calcium phosphide Hydrogen carbide Silver sulphide

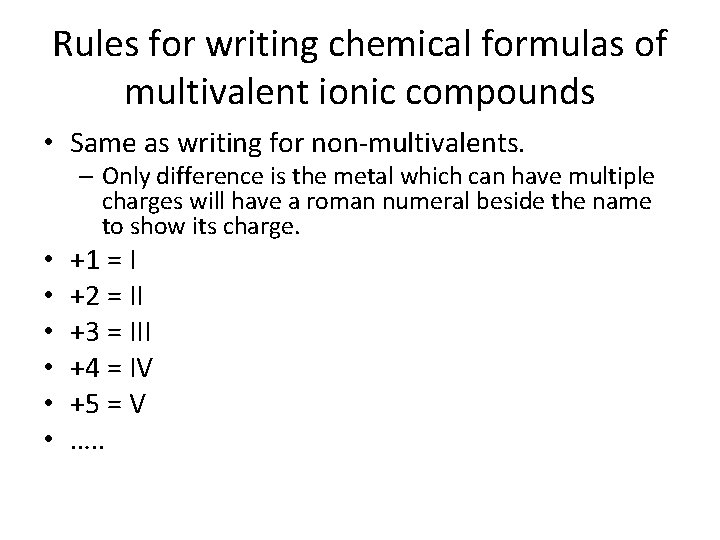
Rules for writing chemical formulas of multivalent ionic compounds • Same as writing for non-multivalents. – Only difference is the metal which can have multiple charges will have a roman numeral beside the name to show its charge. • • • +1 = I +2 = II +3 = III +4 = IV +5 = V …. .

Example - 2 • • • Tin (IV) sulphide Iron (II) phosphide Copper (I) nitride Iron (III) oxide Chromium (III) chloride

Rules for writing chemical formulas of polyatomic ionic compounds • Write as you would naming non-polyatomic ionics • Polyatomic ions are groups of atoms that stay together and carry a charge. They act like a single ion. Treat them like a single ion – NH 4+ – SO 43 - • When you write the formula for polyatomics, they are written as one unit. If there are more than one of the polyatomic ion, you put parentheses around it and state how many. – (NH 4)3 means there are 3 NH 4‘s

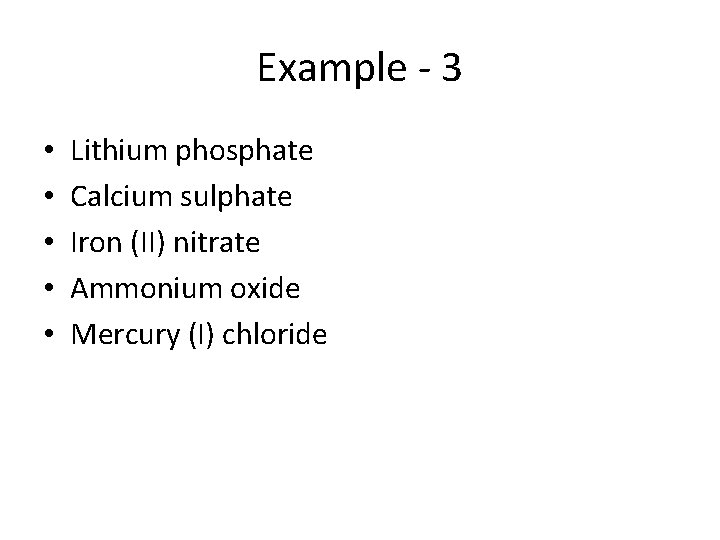
Example - 3 • • • Lithium phosphate Calcium sulphate Iron (II) nitrate Ammonium oxide Mercury (I) chloride

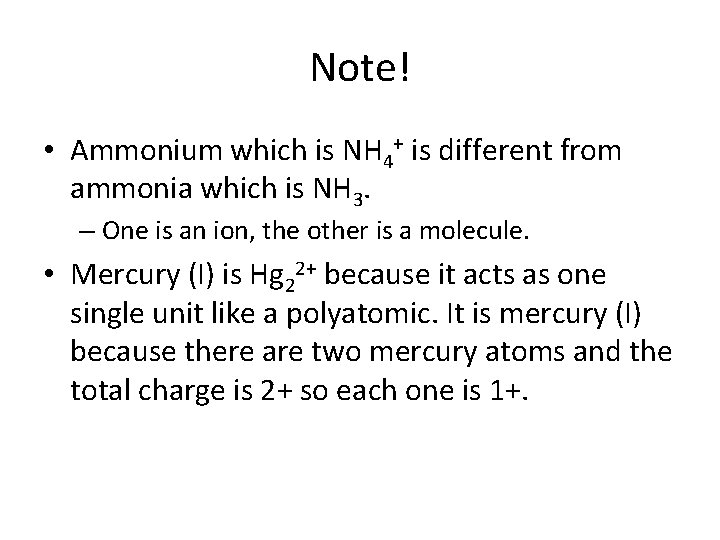
Note! • Ammonium which is NH 4+ is different from ammonia which is NH 3. – One is an ion, the other is a molecule. • Mercury (I) is Hg 22+ because it acts as one single unit like a polyatomic. It is mercury (I) because there are two mercury atoms and the total charge is 2+ so each one is 1+.

Rules for naming chemicals of nonmultivalent ionic compounds • Use the periodic table to find the element symbol • Metal first, non-metal second • The non-metal (anion) drops all its syllables except for the first and ends in IDE – Chloride -> chloride – Oxygen -> oxide – Hydrogen -> hydride • Only the metalloids keep the first two syllables and then ends in IDE • The metal (cation) stays the same in terms of name • DO NOT CAPITALLIZE THE NAME! Only at the start of sentences!

Example - 4 • • • Na. Cl Ca. Cl 2 Ca. O Mg. Cl 2 Li 2 O

Rules for naming chemicals of multivalent ionic compounds • Similar to naming non-multivalents • Look at the charge of the non-metal from the periodic table. It never changes! – Multiply the charge with the number of anions. – This is the total negative charge! • Remember, an ionic compound is neutral so the positive charge must be the opposite positive charge. • Divide the positive charge by the number of cations. This is the charge of each cation. – This is your roman numeral • Write the full name with the roman numeral between the cation and anion in parentheses

Example - 5 • • • Fe 2 S 3 Cu. Cl VCl 3 Cr. I 3 Sn. O 2

Rules for naming chemicals of polyatomic ionic compounds • Similar to the previous two • Just the polyatomic ion does not change ending. Keep it the same! • Sulphate (SO 42 -) is sulphate, not sulphide (S 2 -)

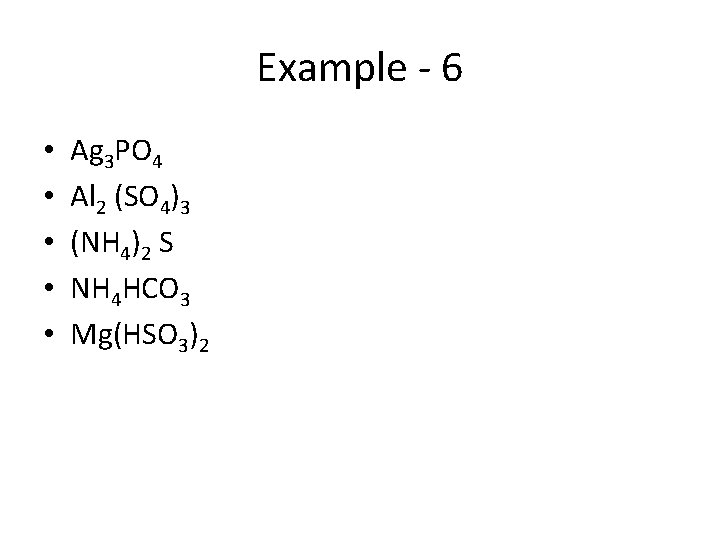
Example - 6 • • • Ag 3 PO 4 Al 2 (SO 4)3 (NH 4)2 S NH 4 HCO 3 Mg(HSO 3)2

Common names • • • H 2 O = water NH 3 = ammonia CH 3 COOH = vinegar Ca. O = lime Na. Cl. O = bleach

Hydrates • Hydrates are ionic compounds that have water molecules attached to it – Even if it is dry! – It is attached as individual molecules after it is dried from an aqueous solution

Rules for writing chemical formulas of hydrates • Write the chemical formula of the ionic as the same as before. • To write the chemical formulas of hydrates, you have to know how many hydrates there are. This is represented by the prefix in front of the word “hydrate” Mono – 1 Hexa – 6 Di – 2 Hepta – 7 Tri – 3 Octa – 8 Tetra – 4 Nona – 9 Penta - 5 Deca - 10

Rules for writing chemical formulas of hydrates • If the name is pentahydrate, you have to write it as 5 H 2 O to state there are 5 water molecules attached. • Octahydrate = 8 H 2 O • You separate the ionic compound from the hydrate with a dot ● (not so big though)

Example - 7 • • • Sodium chloride pentahydrate Iron (III) sulphide hexahydrate Calcium oxide monohydrate Zinc acetate dihydrate Calcium nitrate tetrahydrate

Rules for naming chemicals of hydrates • Same as naming ionic compounds • The coefficient in front of the H 2 O is how many hydrates there are. • You name the ionic compound and then put the prefix for the number of water molecules and then “hydrate”

Example - 8 • • • Fe. Br 3 ● 6 H 2 O Co. F 2 ● 4 H 2 O Na 2 SO 4 ● 10 H 2 O Na 2 S ● 9 H 2 O Al 2 O 3 ● 3 H 2 O

Covalent Compounds • Two or more non-metals put together. No metals involved • Different naming scheme than ionic • Ionic means electrons is the transferring of electrons to the non-metal • Covalent is the sharing of electrons between the non-metals (not equal though) • We use prefixes to tell how many of each elements there are

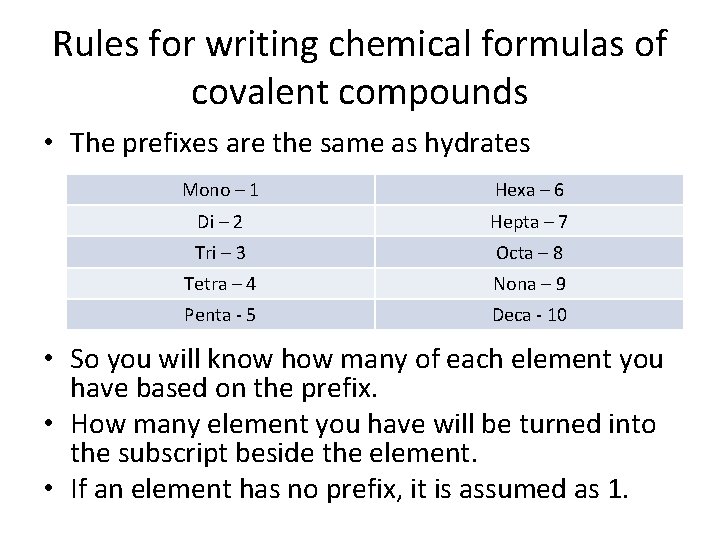
Rules for writing chemical formulas of covalent compounds • The prefixes are the same as hydrates Mono – 1 Hexa – 6 Di – 2 Hepta – 7 Tri – 3 Octa – 8 Tetra – 4 Nona – 9 Penta - 5 Deca - 10 • So you will know how many of each element you have based on the prefix. • How many element you have will be turned into the subscript beside the element. • If an element has no prefix, it is assumed as 1.

Example - 9 • • • Carbon dioxide Carbon monoxide Dinitrogen trioxide Sulphur hexafluoride Dihydrogen monoxide!!! (Very deadly)

Rules for naming of covalent compounds • You keep the first elements name the same, like you do in the metal of ionics • You change the 2 nd element’s ending to IDE like non-metals • You use prefixes to tell how many of each element is present. • You do not need to write MONO if the first element is only 1 as it is assumed.

Example - 10 • • • CO CO 2 P 2 O 3 S 4 N 2 O 3

Naming Acids • All acids start with the element H (hydrogen). – That means even water is an “acid” (more of this in grade 12) • All acids are assumed dissolved in water so they are aqueous solutions • All are assumed to be ionic where the hydrogen acts as the metal • But change the hydrogen to “hydro” if the non -metal is not a polyatomic

Naming Acids • The non-metal name changes • If the non-metal name ends in – IDE turns into IC (hydrogen chloride -> hydrochloric acid) – ATE turns into IC but you drop the hydro (hydrogen sulphate -> sulphuric acid) – ITE turns into OUS and drop the hydro (hydrogen sulphite -> sulphurous acid)

Practice - 1 • • • Page 71 - #4 Page 72 - #5 Page 73 - #6 -7 Page 74 - #8 -9 Page 75 - #14 -163