Nomenclature Chemical Formula type of notation made with


- Slides: 8

Nomenclature

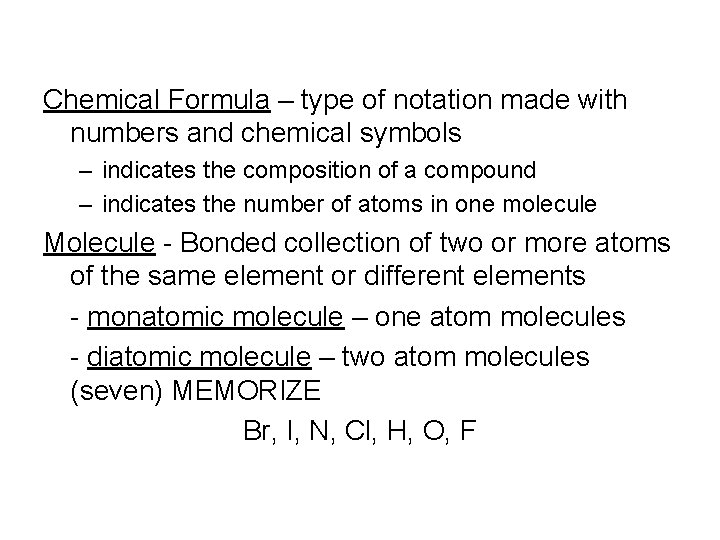
Chemical Formula – type of notation made with numbers and chemical symbols – indicates the composition of a compound – indicates the number of atoms in one molecule Molecule - Bonded collection of two or more atoms of the same element or different elements - monatomic molecule – one atom molecules - diatomic molecule – two atom molecules (seven) MEMORIZE Br, I, N, Cl, H, O, F

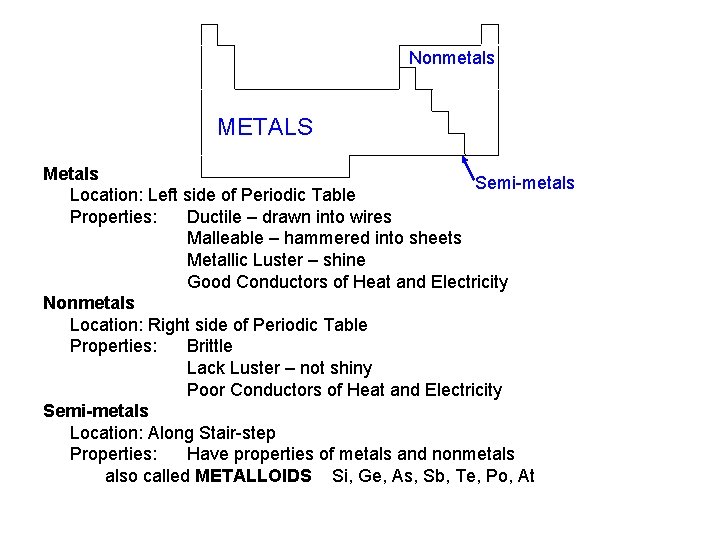
Nonmetals METALS Metals Semi-metals Location: Left side of Periodic Table Properties: Ductile – drawn into wires Malleable – hammered into sheets Metallic Luster – shine Good Conductors of Heat and Electricity Nonmetals Location: Right side of Periodic Table Properties: Brittle Lack Luster – not shiny Poor Conductors of Heat and Electricity Semi-metals Location: Along Stair-step Properties: Have properties of metals and nonmetals also called METALLOIDS Si, Ge, As, Sb, Te, Po, At

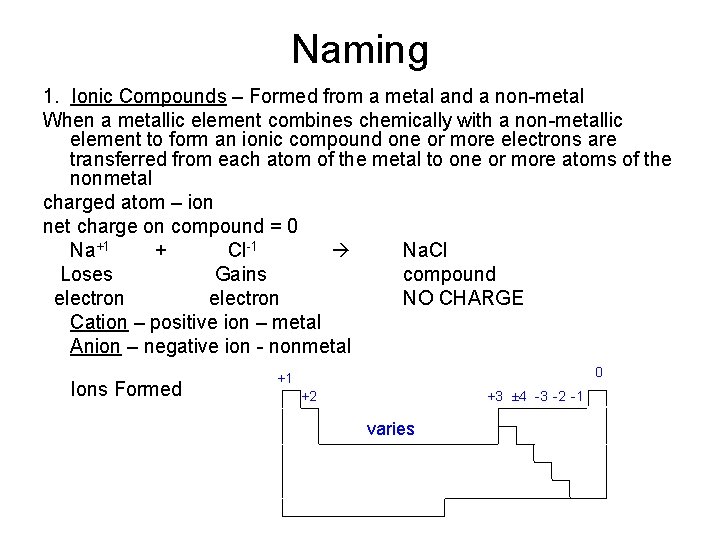
Naming 1. Ionic Compounds – Formed from a metal and a non-metal When a metallic element combines chemically with a non-metallic element to form an ionic compound one or more electrons are transferred from each atom of the metal to one or more atoms of the nonmetal charged atom – ion net charge on compound = 0 Na+1 + Cl-1 Na. Cl Loses Gains compound electron NO CHARGE Cation – positive ion – metal Anion – negative ion - nonmetal Ions Formed 0 +1 +3 ± 4 -3 -2 -1 +2 varies

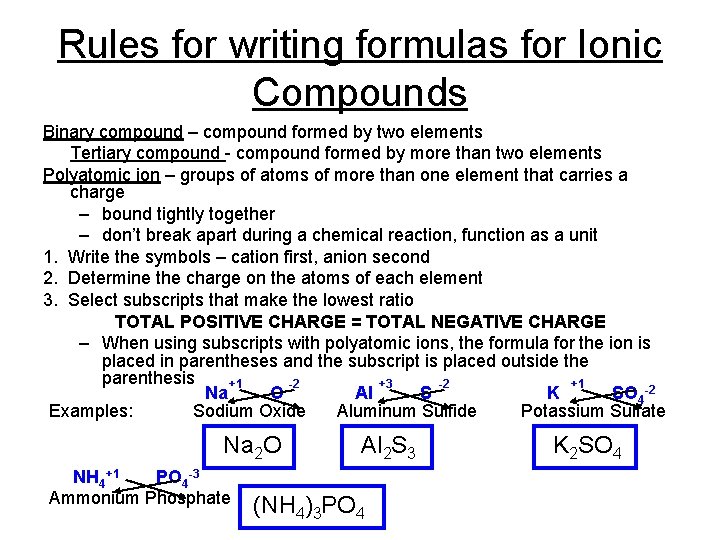
Rules for writing formulas for Ionic Compounds Binary compound – compound formed by two elements Tertiary compound - compound formed by more than two elements Polyatomic ion – groups of atoms of more than one element that carries a charge – bound tightly together – don’t break apart during a chemical reaction, function as a unit 1. Write the symbols – cation first, anion second 2. Determine the charge on the atoms of each element 3. Select subscripts that make the lowest ratio TOTAL POSITIVE CHARGE = TOTAL NEGATIVE CHARGE – When using subscripts with polyatomic ions, the formula for the ion is placed in parentheses and the subscript is placed outside the parenthesis +1 -2 +3 -2 +1 Na O Al S K SO 4 -2 Examples: Sodium Oxide Aluminum Sulfide Potassium Sulfate Na 2 O NH 4+1 PO 4 -3 Ammonium Phosphate Al 2 S 3 (NH 4)3 PO 4 K 2 SO 4

Naming Ionic Compounds Name the Cation first – Name of the metal the anion is the name of the nonmetal altered by adding the suffix -ide to the root word chlorine chloride fluorine fluoride sulfur sulfide oxygen oxide phosphorus phosphide etc… Polyatomic name is not altered Some metals can form more than one kind of ion (Type II) Fe, Cu, Co, Sn, Pb, Hg … Stock System – name the metal followed by a roman numeral in parenthesis. The Roman numeral tells the charge. I, III, IV, V, VI, … Exception: Mercury (I) = Hg 2+2 Examples: Na. Cl = sodium chloride KNO 3 = potassium nitrate Mg. Br 2 = magnesium bromide Cu. O = copper (II) oxide Li 2 SO 4 = lithium sulfate Cu 2 O = copper (I) oxide K 3 N = potassium nitride Sn. S 2 = tin (IV) sulfide

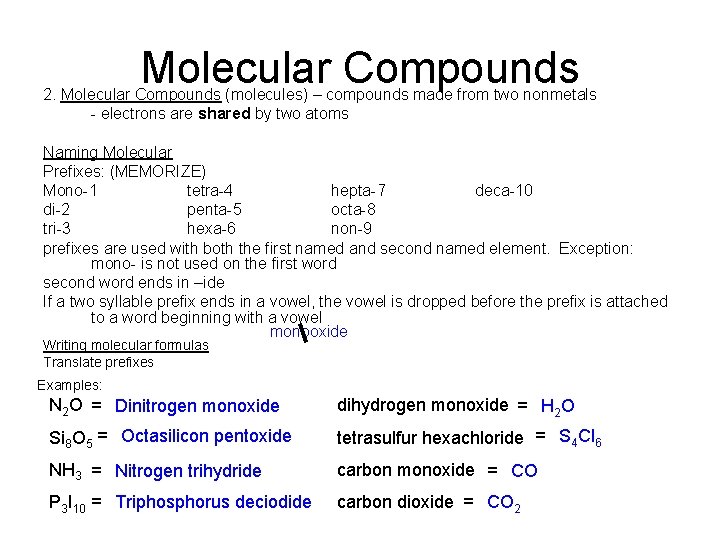
Molecular Compounds 2. Molecular Compounds (molecules) – compounds made from two nonmetals - electrons are shared by two atoms Naming Molecular Prefixes: (MEMORIZE) Mono-1 tetra-4 hepta-7 deca-10 di-2 penta-5 octa-8 tri-3 hexa-6 non-9 prefixes are used with both the first named and second named element. Exception: mono- is not used on the first word second word ends in –ide If a two syllable prefix ends in a vowel, the vowel is dropped before the prefix is attached to a word beginning with a vowel monooxide Writing molecular formulas Translate prefixes Examples: N 2 O = Dinitrogen monoxide dihydrogen monoxide = H 2 O Si 8 O 5 = Octasilicon pentoxide tetrasulfur hexachloride = S 4 Cl 6 NH 3 = Nitrogen trihydride carbon monoxide = CO P 3 I 10 = Triphosphorus deciodide carbon dioxide = CO 2

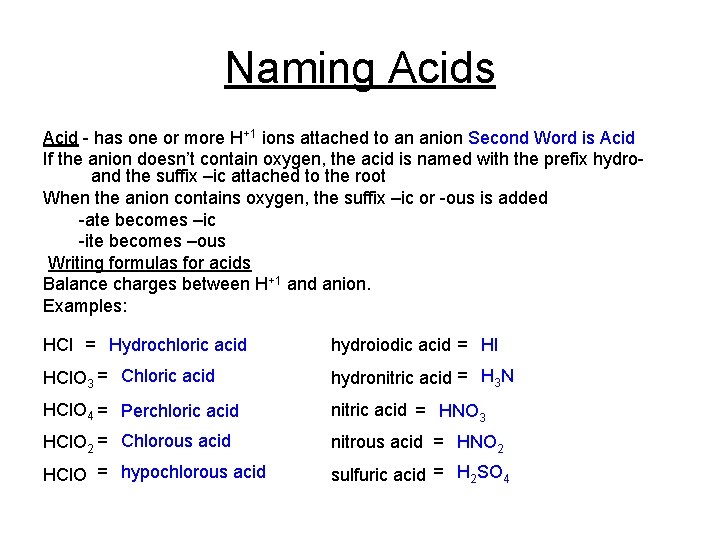
Naming Acids Acid - has one or more H+1 ions attached to an anion Second Word is Acid If the anion doesn’t contain oxygen, the acid is named with the prefix hydroand the suffix –ic attached to the root When the anion contains oxygen, the suffix –ic or -ous is added -ate becomes –ic -ite becomes –ous Writing formulas for acids Balance charges between H+1 and anion. Examples: HCl = Hydrochloric acid hydroiodic acid = HI HCl. O 3 = Chloric acid hydronitric acid = H 3 N HCl. O 4 = Perchloric acid nitric acid = HNO 3 HCl. O 2 = Chlorous acid nitrous acid = HNO 2 HCl. O = hypochlorous acid sulfuric acid = H 2 SO 4