Nomenclature atomic elements elements whose particles are single






- Slides: 24

Nomenclature • atomic elements = elements whose particles are single atoms (i. e. Cu) • molecular elements = elements whose particles are multi-atom molecules (i. e. O 2) • molecular compounds = compounds whose particles are molecules made of only nonmetals (i. e. CO 2) • ionic compounds = compounds whose particles are cations and anions (i. e. Na. Cl) 1

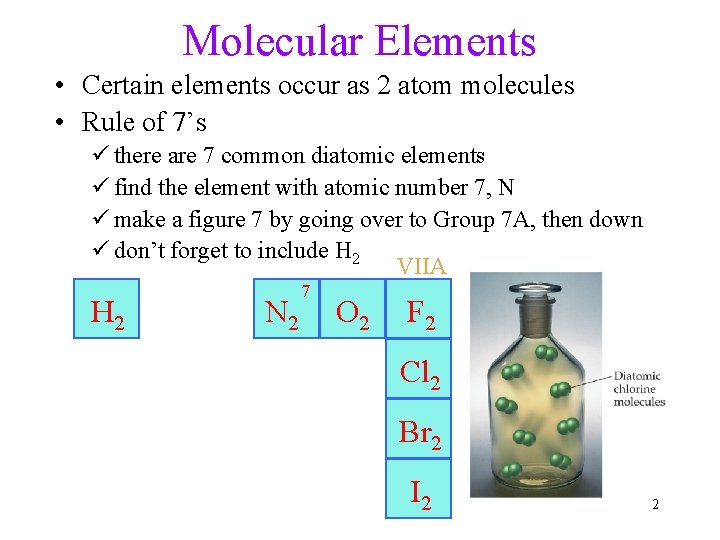
Molecular Elements • Certain elements occur as 2 atom molecules • Rule of 7’s ü there are 7 common diatomic elements ü find the element with atomic number 7, N ü make a figure 7 by going over to Group 7 A, then down ü don’t forget to include H 2 VIIA H 2 N 2 7 O 2 F 2 Cl 2 Br 2 I 2 2

Ionic Compounds anions • cations _______ + _____ • no individual molecule units, instead have a 3 dimensional array of cations and anions made of formula units 3

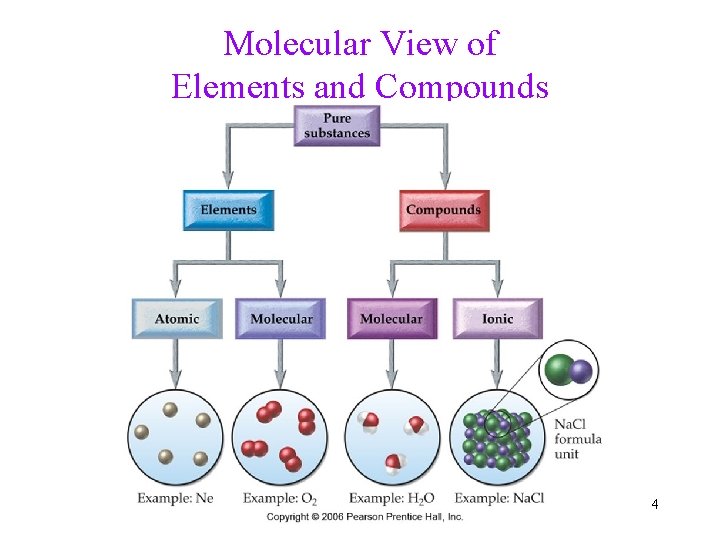
Molecular View of Elements and Compounds 4

Classify each of the following as either an atomic element, molecular compound or ionic compound • aluminum, Al = atomic element • potassium chloride, KCl = ionic compound • chlorine, Cl 2 = molecular element • acetone, C 3 H 6 O = molecular compound • carbon monoxide, CO =molecular compound • cobalt, Co = atomic element 5

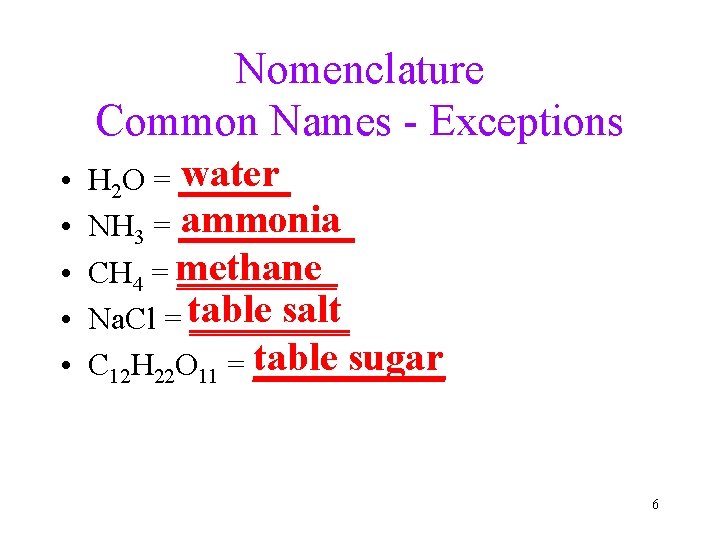
Nomenclature Common Names - Exceptions • • • water H 2 O = _______ ammonia NH 3 = ______ CH 4 = methane _____ salt Na. Cl = table _____ table sugar C 12 H 22 O 11 = ______ 6

• Ionic Nomenclature Major Classes ü metal + nonmetal Ø metal first in formula Ø Binary Ionic ü compounds with polyatomic ions • Molecular ü 2 nonmetals Ø Binary Molecular (or Binary Covalent) ü Acids – formula starts with H Ø though acids are molecular, they behave as ionic when dissolved in water Ø may be binary or oxyacid 7

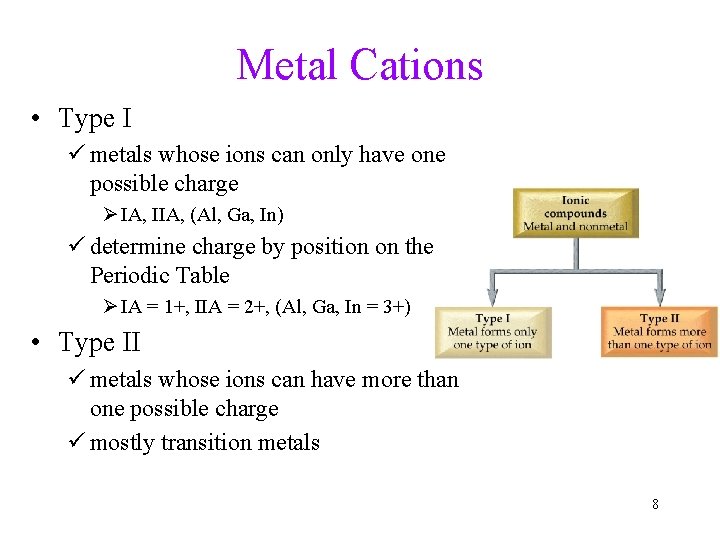
Metal Cations • Type I ü metals whose ions can only have one possible charge Ø IA, IIA, (Al, Ga, In) ü determine charge by position on the Periodic Table Ø IA = 1+, IIA = 2+, (Al, Ga, In = 3+) • Type II ü metals whose ions can have more than one possible charge ü mostly transition metals 8

Type I Metal Cations 9

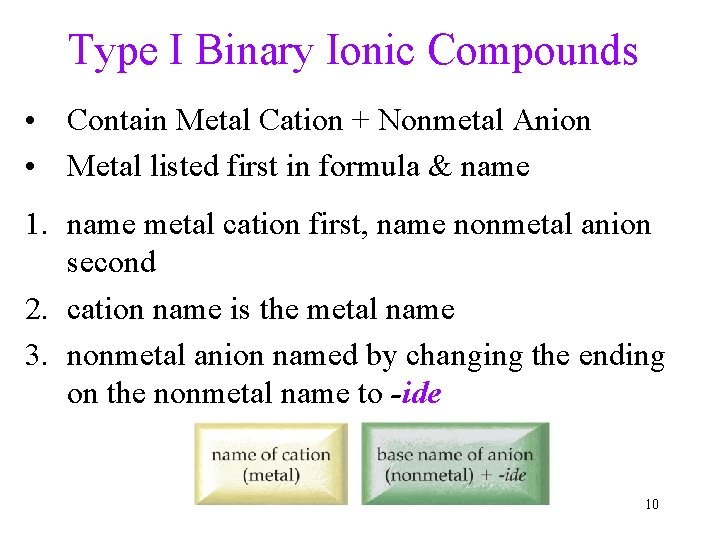
Type I Binary Ionic Compounds • Contain Metal Cation + Nonmetal Anion • Metal listed first in formula & name 1. name metal cation first, name nonmetal anion second 2. cation name is the metal name 3. nonmetal anion named by changing the ending on the nonmetal name to -ide 10

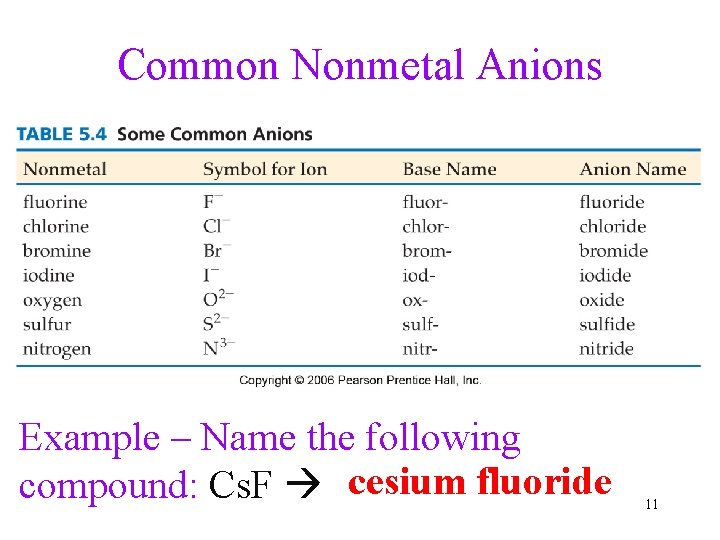
Common Nonmetal Anions Example – Name the following compound: Cs. F cesium fluoride 11

Type II Binary Ionic Compounds • • Contain Metal Cation + Nonmetal Anion Metal listed first in formula & name 1. name metal cation first, name nonmetal anion second 2. metal cation name is the metal name followed by a Roman Numeral in parentheses to indicate its charge 3. nonmetal anion named by changing the ending on the nonmetal name to -ide 12

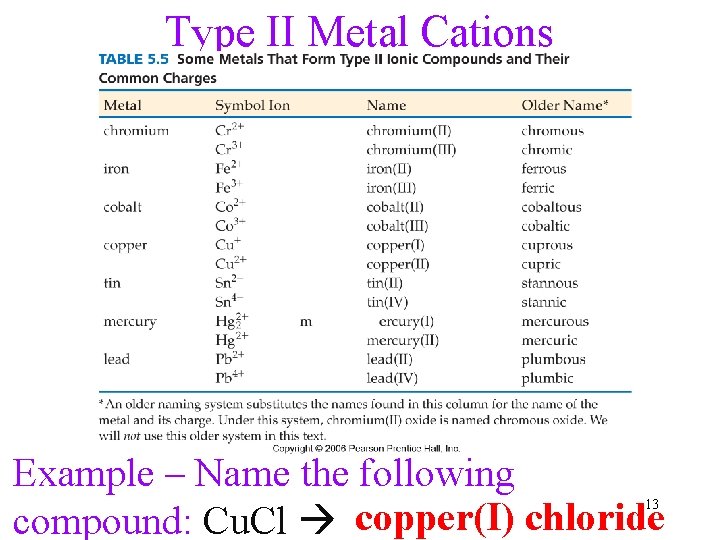
Type II Metal Cations Example – Name the following compound: Cu. Cl copper(I) chloride 13

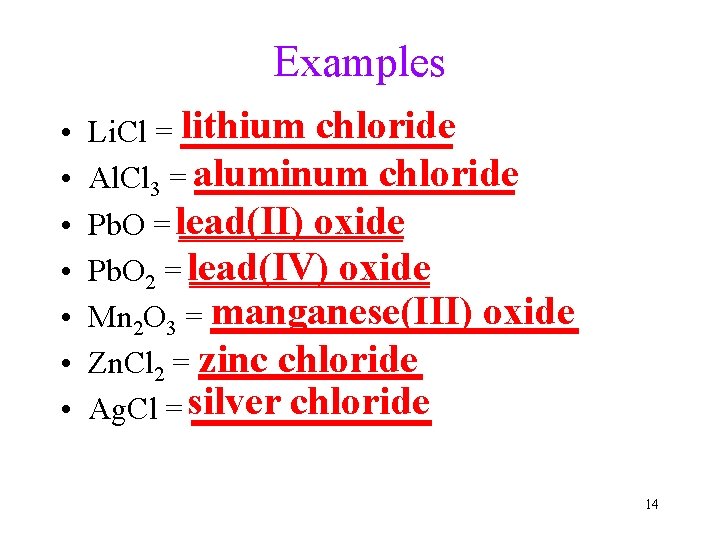
Examples • • lithium chloride Li. Cl = _________ chloride Al. Cl 3 = aluminum __________ oxide Pb. O = lead(II) _______ oxide Pb. O 2 = lead(IV) ________ manganese(III) oxide Mn 2 O 3 = ____________ zinc chloride Zn. Cl 2 = _______ chloride Ag. Cl = silver ________ 14

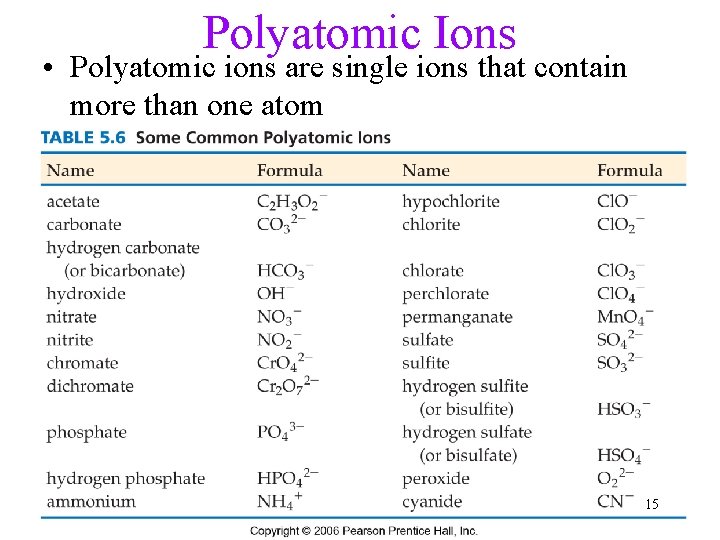
Polyatomic Ions • Polyatomic ions are single ions that contain more than one atom 15

Example – Name the following compounds: Na 2 SO 4 sodium sulfate Fe(NO 3)3 iron(III) nitrate 16

Binary Molecular Compounds of 2 Nonmetals 1. Name first element in formula first ü use the full name of the element 2. Name the second element in the formula with an -ide ü as if it were an anion, however, remember these compounds do not contain ions! 3. Use a prefix in front of each name to indicate the number of atoms a) Do not use the prefix mono- on the first element 17

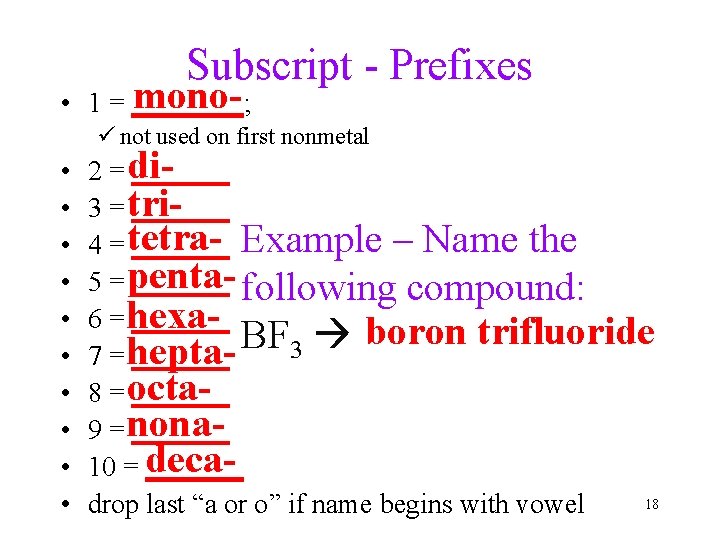
Subscript - Prefixes mono • 1 = ____; ü not used on first nonmetal • • • 2 = di_______ 3 = tri_______ 4 = tetra_______ Example – Name the 5 = penta_______ following compound: 6 = hexa_______ boron trifluoride BF 3 7 = hepta_______ 8 = octa_______ 9 = nona_______ 10 = deca_______ 18 drop last “a or o” if name begins with vowel

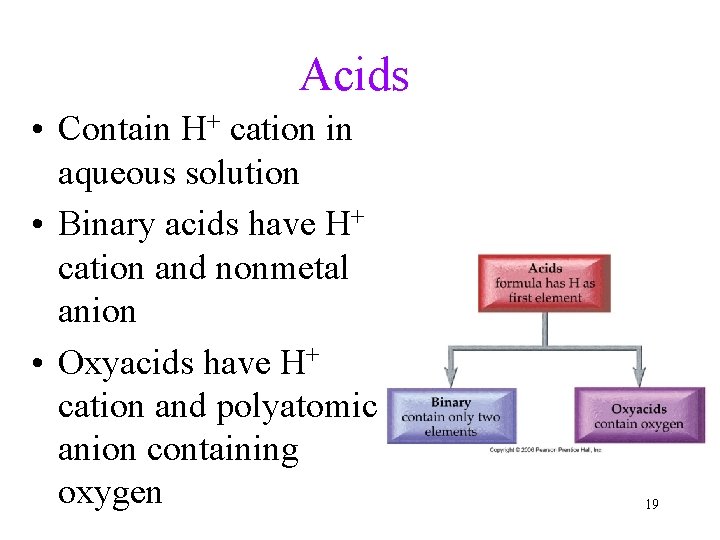
Acids • Contain H+ cation in aqueous solution • Binary acids have H+ cation and nonmetal anion • Oxyacids have H+ cation and polyatomic anion containing oxygen 19

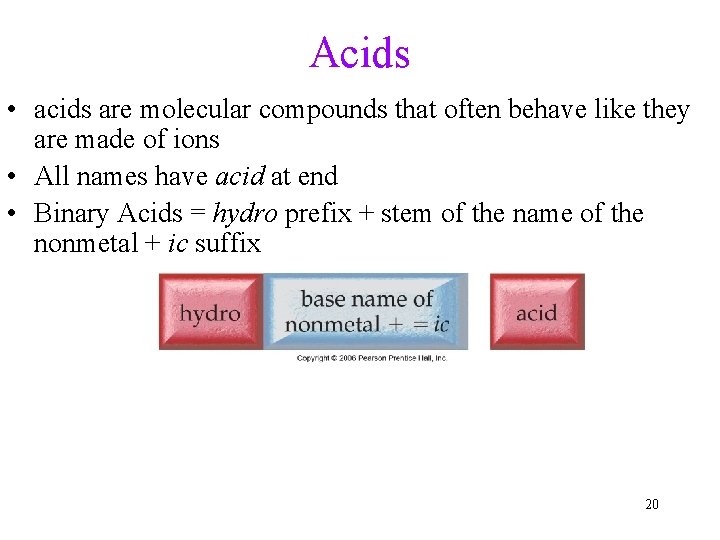
Acids • acids are molecular compounds that often behave like they are made of ions • All names have acid at end • Binary Acids = hydro prefix + stem of the name of the nonmetal + ic suffix 20

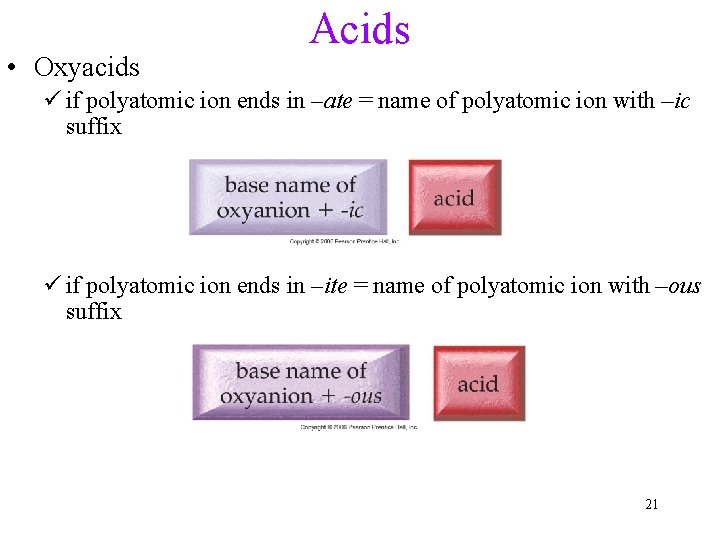
• Oxyacids Acids ü if polyatomic ion ends in –ate = name of polyatomic ion with –ic suffix ü if polyatomic ion ends in –ite = name of polyatomic ion with –ous suffix 21

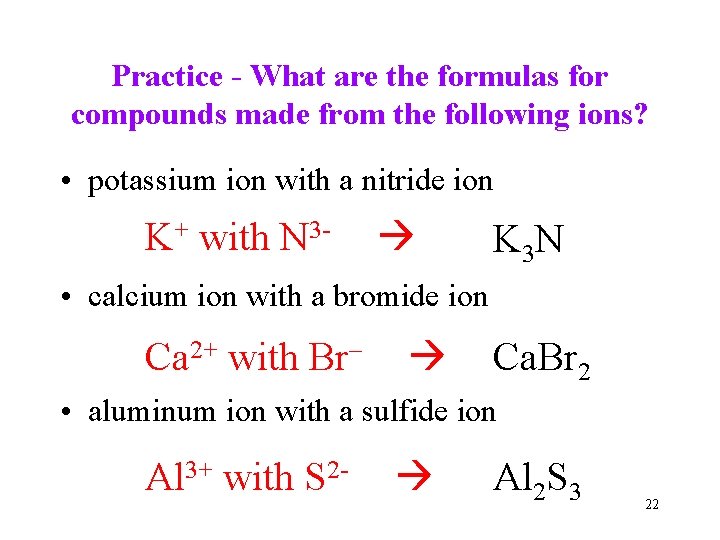
Practice - What are the formulas for compounds made from the following ions? • potassium ion with a nitride ion K+ with N 3 - K 3 N • calcium ion with a bromide ion Ca 2+ with Br– Ca. Br 2 • aluminum ion with a sulfide ion Al 3+ with S 2 - Al 2 S 3 22

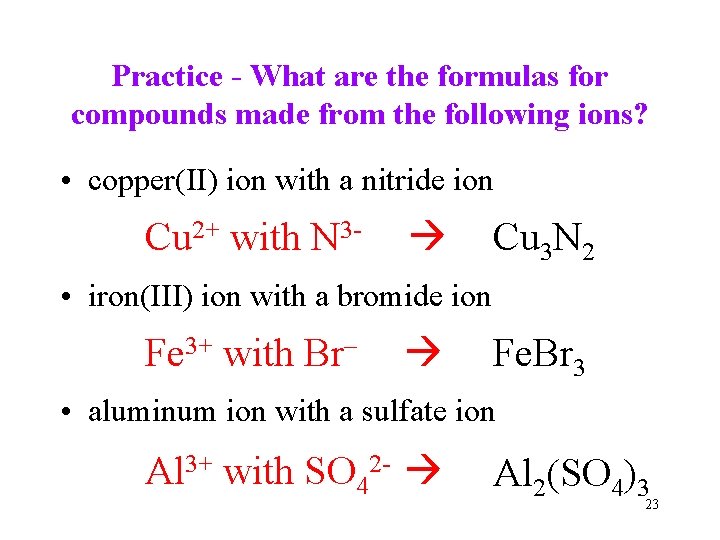
Practice - What are the formulas for compounds made from the following ions? • copper(II) ion with a nitride ion Cu 2+ with N 3 - Cu 3 N 2 • iron(III) ion with a bromide ion Fe 3+ with Br– Fe. Br 3 • aluminum ion with a sulfate ion Al 3+ with SO 42 - Al 2(SO 4)3 23

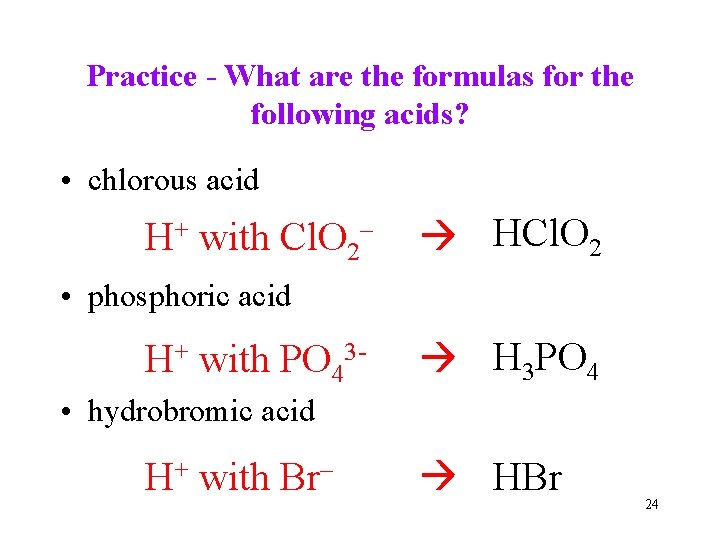
Practice - What are the formulas for the following acids? • chlorous acid H+ with Cl. O 2– HCl. O 2 • phosphoric acid H+ with PO 43 - H 3 PO 4 • hydrobromic acid H+ with Br– HBr 24