Nobles gases and Isotopes IGCSE Combined Science Noble
Nobles gases and Isotopes IGCSE Combined Science
Noble Gas Starter What is the electron arrangement for He ? 2 What is the electron arrangement for Ne ? 2, 8 For Ar ? 2, 8. 8 What do these arrangements have in common ?
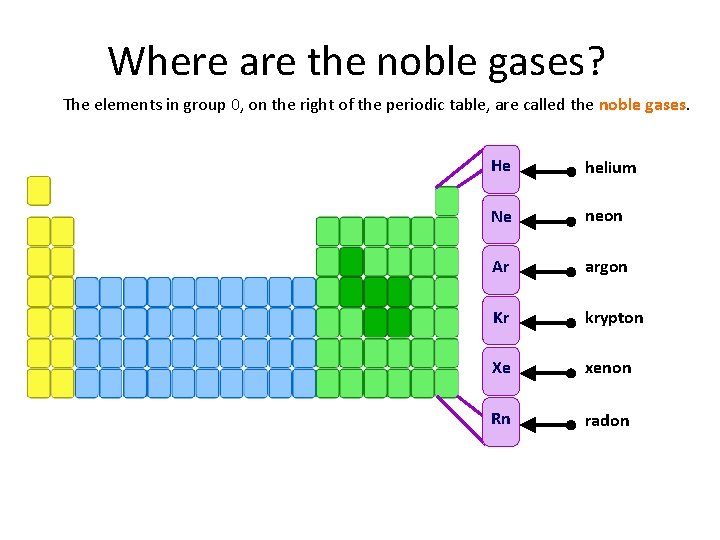
Where are the noble gases? The elements in group 0, on the right of the periodic table, are called the noble gases. He helium Ne neon Ar argon Kr krypton Xe xenon Rn radon

Why are they called the ‘noble gases’? The noble gases all form colourless gases at room temperature. They are all very unreactive. Noble gases were originally called ‘inert gases’, as they were thought not to react with anything. Then in 1962, a British chemist, Neil Bartlett, made a compound with xenon. The name was changed to ‘noble gases’ as they were considered similar to the very unreactive precious metals gold and platinum, which are sometimes called ‘noble’ metals. Now only neon and helium have not yet been made to form compounds.

How does electron structure affect reactivity? All noble gases have full outer electron shells and do not need to gain, lose or share electrons. This means that: l The noble gases are very stable and the most unreactive (or inert) of all the elements. All of the noble gases are similarly unreactive, up and down the group. helium 2 neon 2, 8 l They do not normally form bonds with other elements. l They are monatomic, which means they exist as individual atoms. Most other gases are diatomic. argon 2, 8, 8
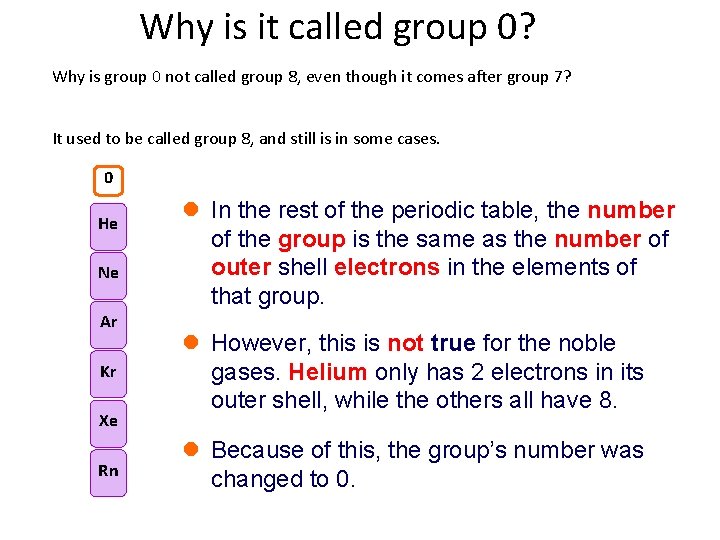
Why is it called group 0? Why is group 0 not called group 8, even though it comes after group 7? It used to be called group 8, and still is in some cases. 8 0 He Ne Ar Kr Xe Rn l In the rest of the periodic table, the number of the group is the same as the number of outer shell electrons in the elements of that group. l However, this is not true for the noble gases. Helium only has 2 electrons in its outer shell, while the others all have 8. l Because of this, the group’s number was changed to 0.
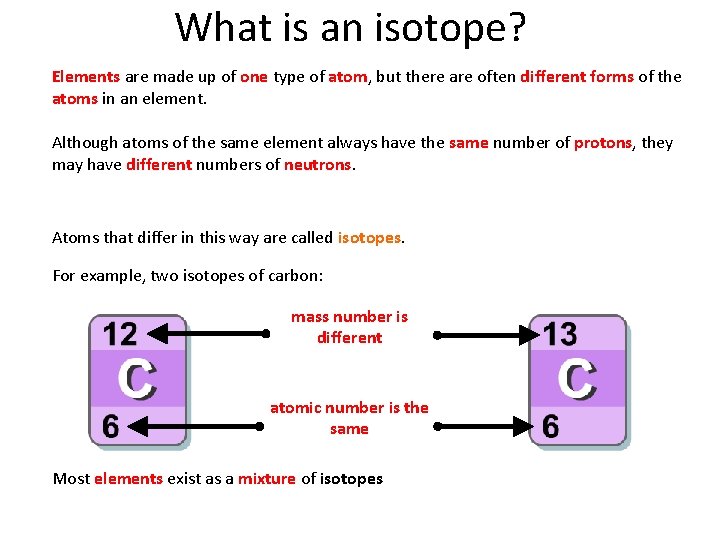
What is an isotope? Elements are made up of one type of atom, but there are often different forms of the atoms in an element. Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that differ in this way are called isotopes. For example, two isotopes of carbon: mass number is different atomic number is the same Most elements exist as a mixture of isotopes
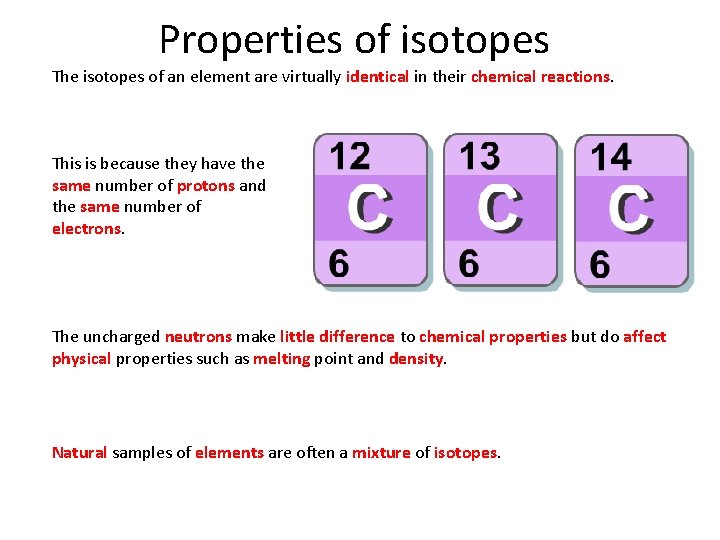
Properties of isotopes The isotopes of an element are virtually identical in their chemical reactions. This is because they have the same number of protons and the same number of electrons. The uncharged neutrons make little difference to chemical properties but do affect physical properties such as melting point and density. Natural samples of elements are often a mixture of isotopes.
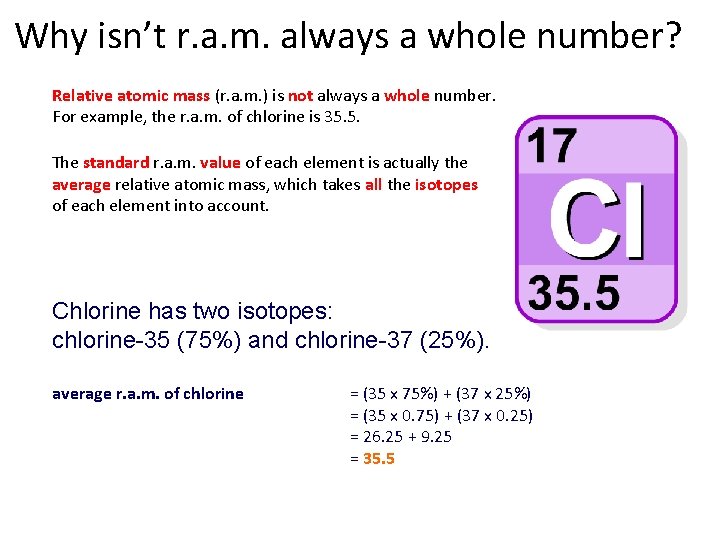
Why isn’t r. a. m. always a whole number? Relative atomic mass (r. a. m. ) is not always a whole number. For example, the r. a. m. of chlorine is 35. 5. The standard r. a. m. value of each element is actually the average relative atomic mass, which takes all the isotopes of each element into account. Chlorine has two isotopes: chlorine-35 (75%) and chlorine-37 (25%). average r. a. m. of chlorine = (35 x 75%) + (37 x 25%) = (35 x 0. 75) + (37 x 0. 25) = 26. 25 + 9. 25 = 35. 5
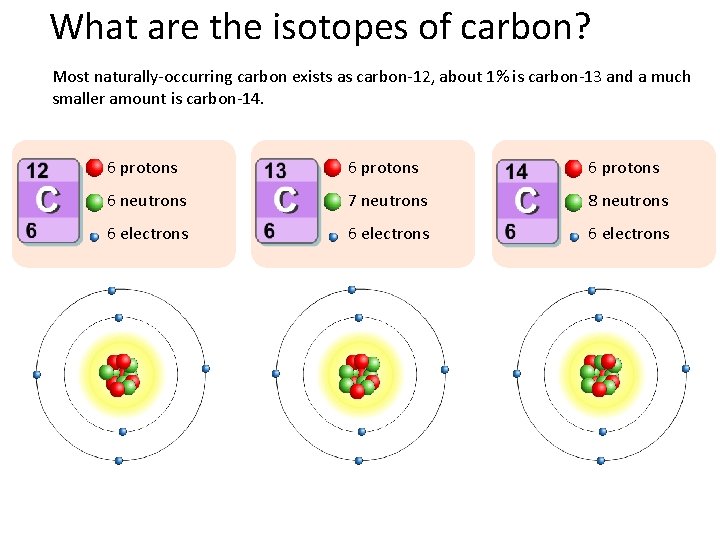
What are the isotopes of carbon? Most naturally-occurring carbon exists as carbon-12, about 1% is carbon-13 and a much smaller amount is carbon-14. 6 protons 6 neutrons 7 neutrons 8 neutrons 6 electrons
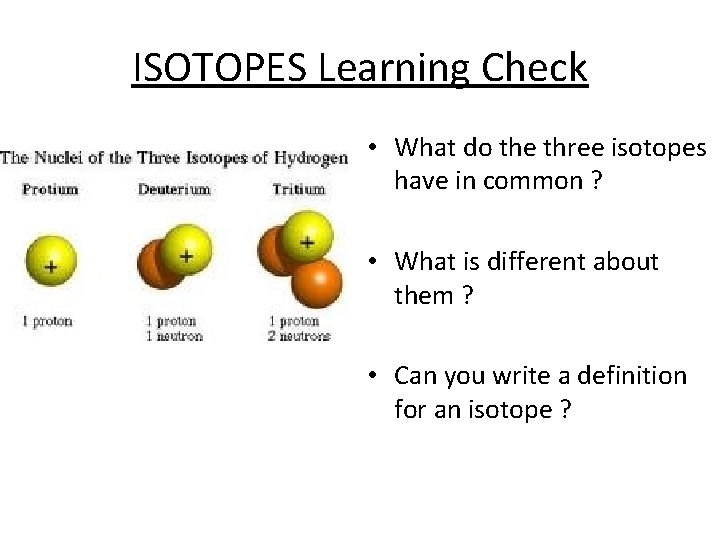
ISOTOPES Learning Check • What do the three isotopes have in common ? • What is different about them ? • Can you write a definition for an isotope ?
- Slides: 11