Noble Gases Group 18 elements He Ne Ar

: -: -: - Noble Gases (Group 18 elements) He, Ne, Ar, Kr, Xe, Rn

Why termed as noble gases? �Chemically unreactive/least reactive/inactive nature. �Form few compounds. �Completely filled valence shell orbitals. ns 2 np 6 except He ns 2 �Closed shell structures
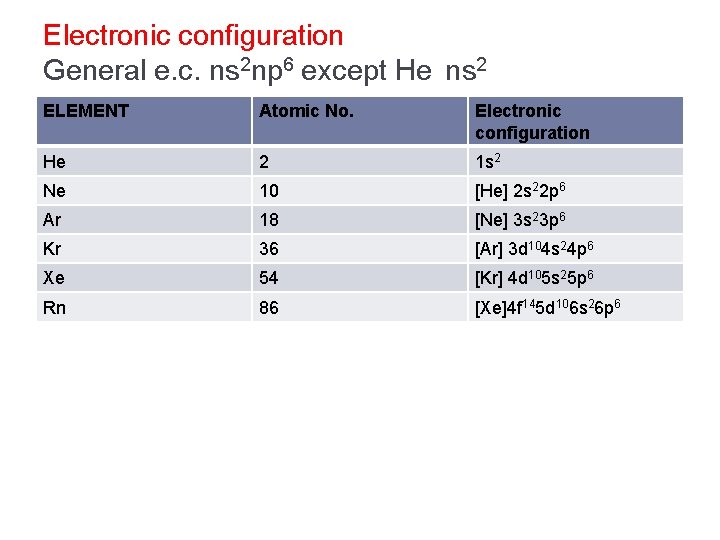
Electronic configuration General e. c. ns 2 np 6 except He ns 2 ELEMENT Atomic No. Electronic configuration He 2 1 s 2 Ne 10 [He] 2 s 22 p 6 Ar 18 [Ne] 3 s 23 p 6 Kr 36 [Ar] 3 d 104 s 24 p 6 Xe 54 [Kr] 4 d 105 s 25 p 6 Rn 86 [Xe]4 f 145 d 106 s 26 p 6

� Atomic size: - increases down the group due to increase in number of shells. � Ionization enthalpy: -very high due to completely filled valence shell orbitals. Decreases down the group with increase in atomic size. � Electron gain enthalpy: - large positive values due to completely filled valence shell orbitals. No tendency to accept the electrons. They have stable electronic configurations. � Melting and Boiling points: - very low due to weak dispersion forces. � Helium has the lowest boiling point (4. 2 K) of any known substance. It has an unusual property of diffusing through most commonly used laboratory materials such as rubber, glass or plastics.

Reactivity: -least reactive? Due to� Completely filled valence shell / closed shell structures. � Very high ionization enthalpy. � Large positive electron gain enthalpy.
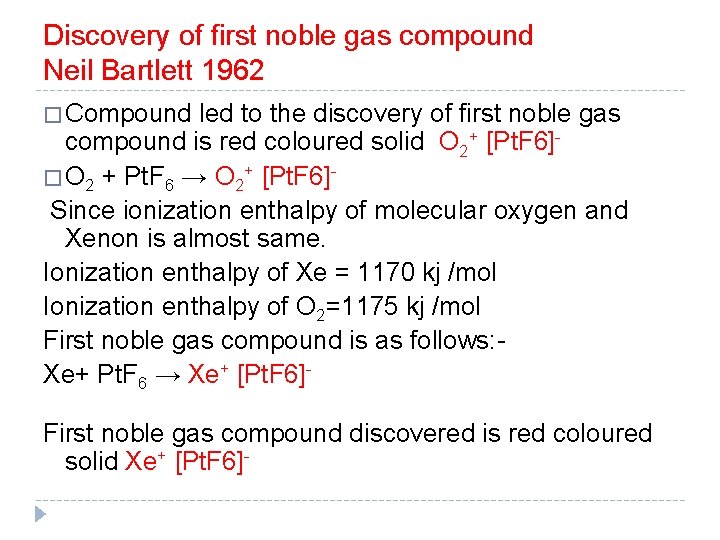
Discovery of first noble gas compound Neil Bartlett 1962 � Compound led to the discovery of first noble gas compound is red coloured solid O 2+ [Pt. F 6]� O 2 + Pt. F 6 → O 2+ [Pt. F 6]Since ionization enthalpy of molecular oxygen and Xenon is almost same. Ionization enthalpy of Xe = 1170 kj /mol Ionization enthalpy of O 2=1175 kj /mol First noble gas compound is as follows: Xe+ Pt. F 6 → Xe+ [Pt. F 6]First noble gas compound discovered is red coloured solid Xe+ [Pt. F 6]-
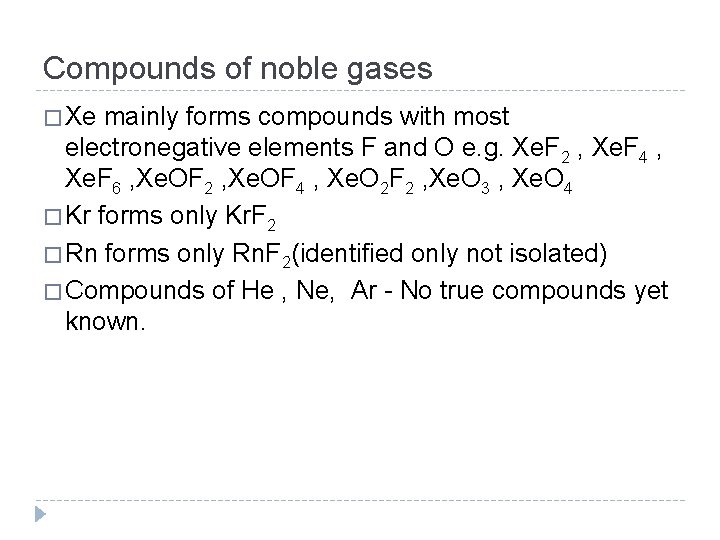
Compounds of noble gases � Xe mainly forms compounds with most electronegative elements F and O e. g. Xe. F 2 , Xe. F 4 , Xe. F 6 , Xe. OF 2 , Xe. OF 4 , Xe. O 2 F 2 , Xe. O 3 , Xe. O 4 � Kr forms only Kr. F 2 � Rn forms only Rn. F 2(identified only not isolated) � Compounds of He , Ne, Ar - No true compounds yet known.

Preparation of compounds of Xe � Xe (g) + F 2 (g) �� 673 K, 1 bar → Xe. F 2(s) � (xenon in excess) � Xe (g) + 2 F 2 (g) �� 873 K, 7 bar �→Xe. F 4(s) � (1: 5 ratio) � Xe (g) + 3 F 2 (g) �� 573 K, 60� 70 bar → Xe. F 6(s) � (1: 20 ratio) � Xe. F 6 can also be prepared by the interaction of Xe. F 4 and O 2 F 2 at 143 K. � Xe. F 4 + O 2 F 2 → Xe. F 6 + O 2 � Xe. F 2, Xe. F 4 and Xe. F 6 are colourless , powerful fluorinating agents.
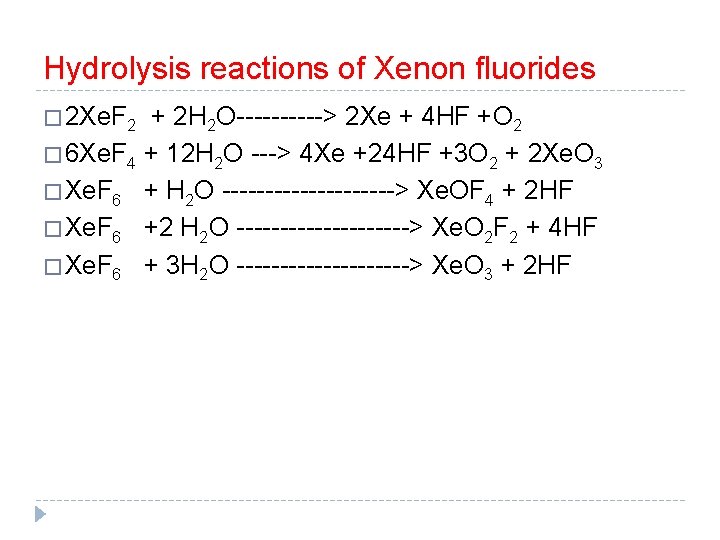
Hydrolysis reactions of Xenon fluorides � 2 Xe. F 2 + 2 H 2 O-----> 2 Xe + 4 HF +O 2 � 6 Xe. F 4 + 12 H 2 O ---> 4 Xe +24 HF +3 O 2 + 2 Xe. O 3 � Xe. F 6 + H 2 O ----------> Xe. OF 4 + 2 HF � Xe. F 6 +2 H 2 O ----------> Xe. O 2 F 2 + 4 HF � Xe. F 6 + 3 H 2 O ----------> Xe. O 3 + 2 HF
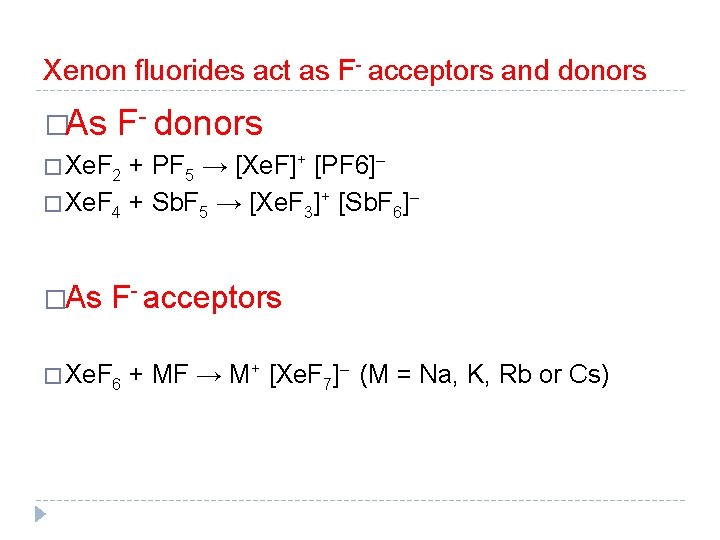
Xenon fluorides act as F- acceptors and donors �As F- donors � Xe. F 2 + PF 5 → [Xe. F]+ [PF 6]– � Xe. F 4 + Sb. F 5 → [Xe. F 3]+ [Sb. F 6]– �As F- acceptors � Xe. F 6 + MF → M+ [Xe. F 7]– (M = Na, K, Rb or Cs)
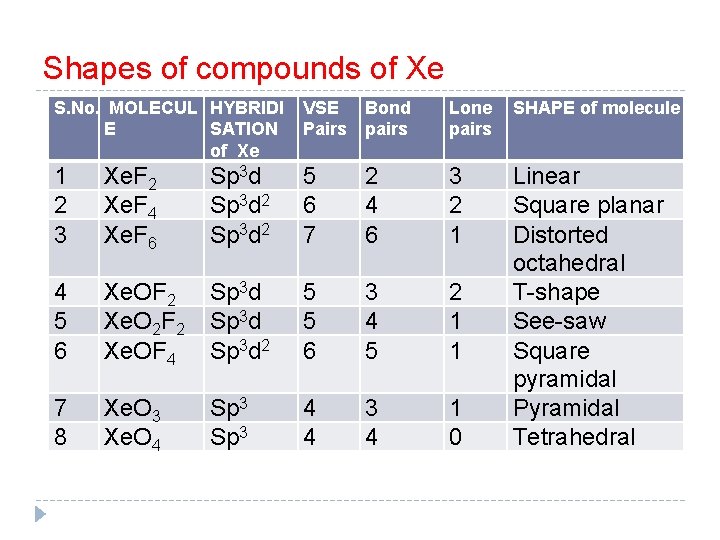
Shapes of compounds of Xe S. No. MOLECUL HYBRIDI E SATION of Xe VSE Pairs Bond pairs Lone pairs SHAPE of molecule 1 2 3 Xe. F 2 Xe. F 4 Xe. F 6 Sp 3 d 2 5 6 7 2 4 6 3 2 1 4 5 6 Xe. OF 2 Xe. O 2 F 2 Xe. OF 4 Sp 3 d 2 5 5 6 3 4 5 2 1 1 7 8 Xe. O 3 Xe. O 4 Sp 3 4 4 3 4 1 0 Linear Square planar Distorted octahedral T-shape See-saw Square pyramidal Pyramidal Tetrahedral
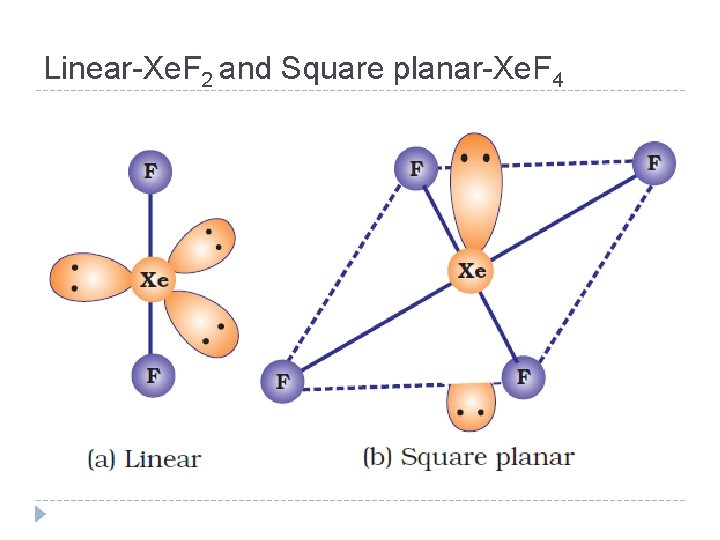
Linear-Xe. F 2 and Square planar-Xe. F 4
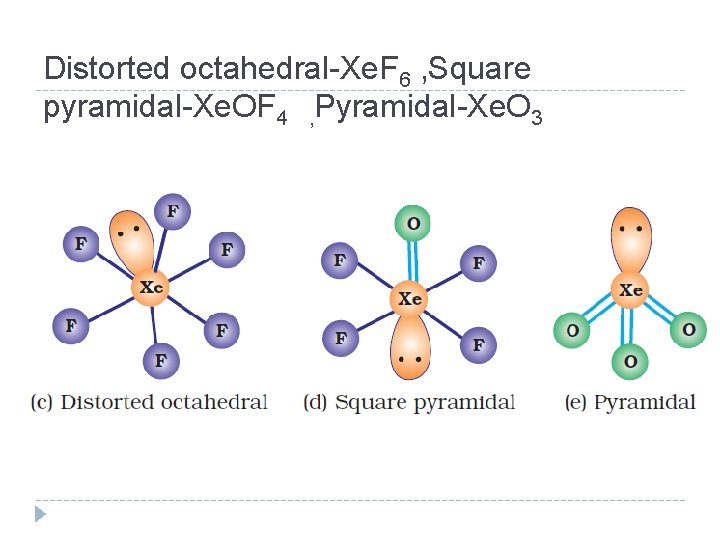
Distorted octahedral-Xe. F 6 , Square pyramidal-Xe. OF 4 , Pyramidal-Xe. O 3
Uses of noble gases Helium : - is a non-inflammable and light gas. Hence, it is used in filling balloons for meteorological observations. It is also used in gas-cooled nuclear reactors. Liquid helium (b. p. 4. 2 K) finds use as cryogenic agent for carrying out various experiments at low temperatures. It is used to produce and sustain powerful superconducting magnets which form an essential part of modern NMR spectrometers and Magnetic Resonance Imaging (MRI) systems for clinical diagnosis. It is used as a diluent for oxygen in modern diving apparatus because of its very low solubility in blood.

Neon , Argon, Krypyon , Xenon Neon : - used in discharge tubes and fluorescent bulbs for advertisement display purposes. Neon bulbs are used in botanical gardens and in green houses. Argon : - is used mainly to provide an inert atmosphere in high temperature metallurgical processes (arc welding of metals or alloys) and for filling electric bulbs. It is also used in the laboratory for handling substances that are air-sensitive. Krypton and Xenon : - There are no significant uses of Xenon and Krypton. They are used in light bulbs designed for special purposes.

THANKS � SANJEEV KUMAR � PGT CHEMISTRY � K. V. YOL CANTT � GURUGRAM REGION
- Slides: 16