Noble Gas Configuration Warm up Electron Configuration Review

Noble Gas Configuration

Warm up: Electron Configuration Review Element E-Configuration Phosphorous Arsenic Rubidium Practice on your desk with the Neon Expo markers!

Element e- configuration Phosphorous 1 s 2 2 p 6 3 s 2 3 p 3 Arsenic 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 3 Rubidium 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 1
Now that you have mastered electron Configuration…. . There is a short cut Ne Xe NOBLE GAS NOTATION Kr Rn Ar
Where do we find the Noble Gasses on the periodic Table?

How does it work? 1. Determine the noble gas that comes just BEFORE (lower atomic #)the element in which you are trying to complete it’s electron configuration 2. Put that noble gas’s symbol in brackets to start the configuration 3. Start the configuration from there, not from the beginning

try this: Iodine: Long format: 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 5 Noble Gas Format: [Kr] 5 s 24 d 105 p 5 Oh! That’s much easier! I like that much better!
Why use Noble Gas Configuration? It simplifies the written expression of an elements e- configuration

Practice: Element Magnesium Sulfur Cobalt Molybdenim Barium Noble Gas Configuration

Your Turn: PUT THIS IN YOUR NOTES Element Boron Sodium Calcium Copper Francium Noble Gas Configuration
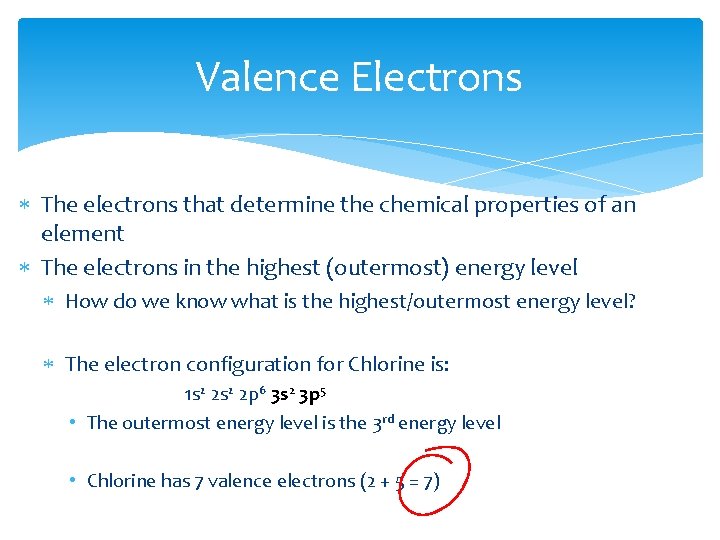
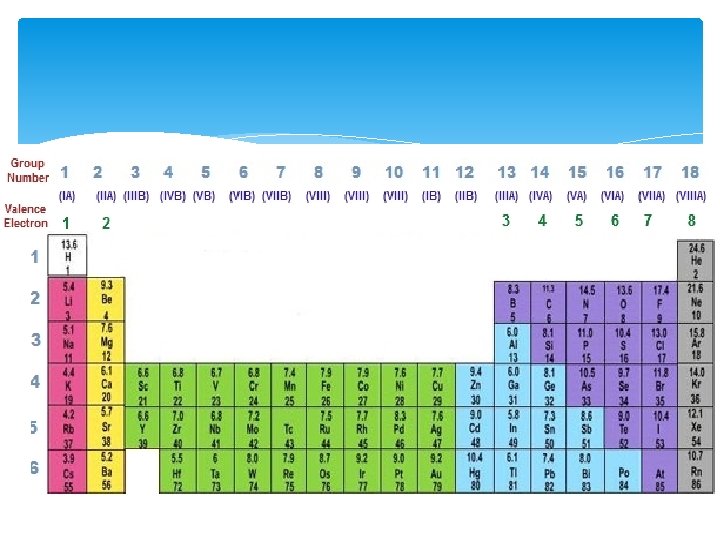
Valence Electrons The electrons that determine the chemical properties of an element The electrons in the highest (outermost) energy level How do we know what is the highest/outermost energy level? The electron configuration for Chlorine is: 1 s 2 2 p 6 3 s 2 3 p 5 • The outermost energy level is the 3 rd energy level • Chlorine has 7 valence electrons (2 + 5 = 7)
Lewis Dot Structure A model that uses electron-dot structures to show electrons are arranged in molecules. Shows the valence electrons around the element symbol What is an element symbol? Aluminum has _____ valence electrons Imagine aluminum symbol with a box around it Al *Each side of the box can only hold 2 electrons *Put 1 dot (to represent 1 electron) on each side before adding a second dot to that side of the box – single electrons must occupy each orbital before additional electrons may occupy the same orbital. So the final product looks like this: Al

Give Sulfur a try: How many valence electrons does Sulfur have? ______ S
Your turn: Complete these in your notes Draw the Lewis Dot Structure for the following elements: Element Lithium Nitrogen Calcium Bromine Carbon Selenium Symbol and number of valence electrons Lewis Dot Structure
- Slides: 15