NMR Structure Determination With The NMR Assignments and

- Slides: 69

NMR Structure Determination With The NMR Assignments and Molecular Modeling Tools in Hand: • All we need are the experimental constraints Distance constraints between atoms is the primary structure determination factor. t Dihedral angles are also an important structural constraint t What Structural Information is available from an NMR spectra? How is it Obtained? How is it Interpreted?

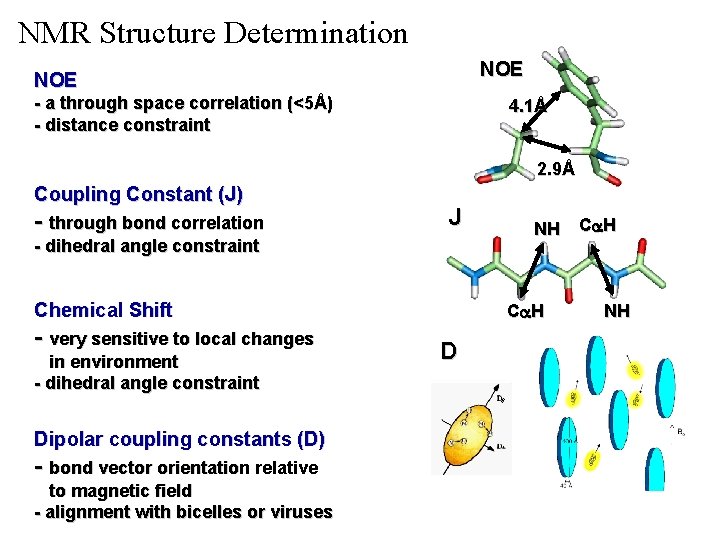
NMR Structure Determination NOE - a through space correlation (<5Å) - distance constraint 4. 1Å 2. 9Å Coupling Constant (J) - through bond correlation J - dihedral angle constraint Chemical Shift - very sensitive to local changes in environment - dihedral angle constraint Dipolar coupling constants (D) - bond vector orientation relative to magnetic field - alignment with bicelles or viruses NH Ca H D NH

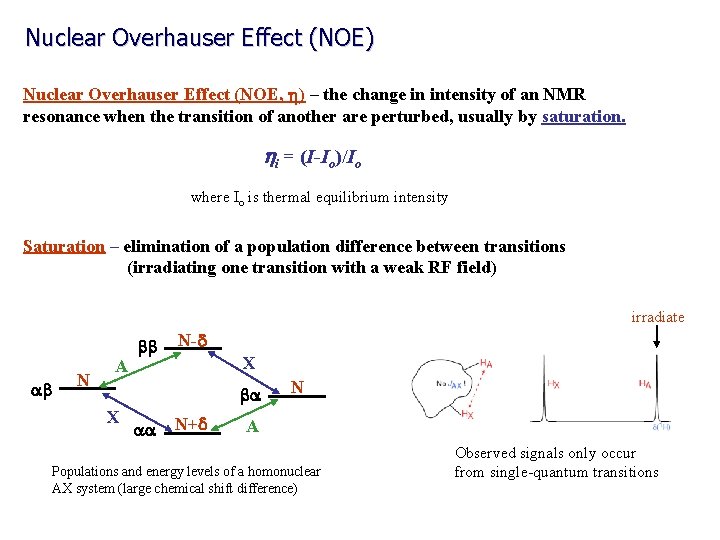
Nuclear Overhauser Effect (NOE) Nuclear Overhauser Effect (NOE, h) – the change in intensity of an NMR resonance when the transition of another are perturbed, usually by saturation. hi = (I-Io)/Io where Io is thermal equilibrium intensity Saturation – elimination of a population difference between transitions (irradiating one transition with a weak RF field) irradiate ab N A bb N-d X ba X aa N+d N A Populations and energy levels of a homonuclear AX system (large chemical shift difference) Observed signals only occur from single-quantum transitions

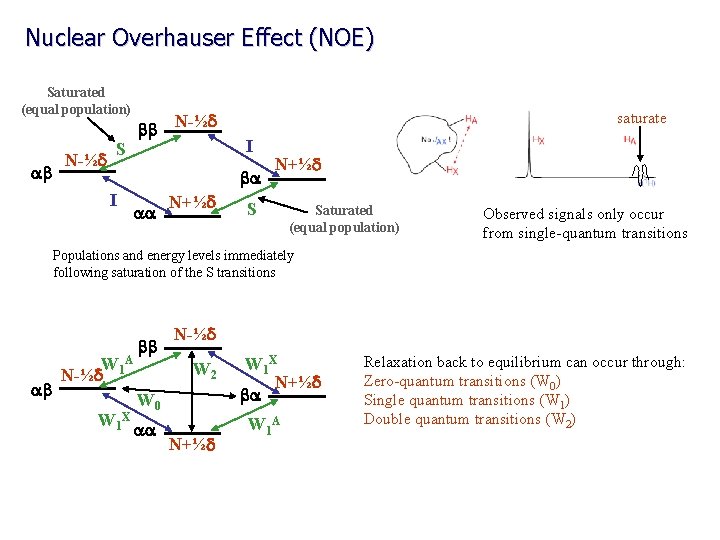
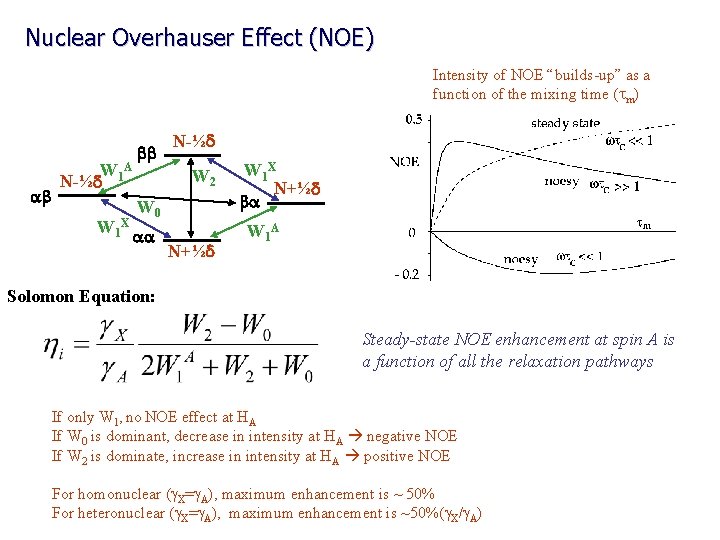
Nuclear Overhauser Effect (NOE) Saturated (equal population) ab N-½d S bb N-½d saturate I ba I aa N+½d S Saturated (equal population) Observed signals only occur from single-quantum transitions Populations and energy levels immediately following saturation of the S transitions ab W N-½d 1 A W 1 X bb N-½d W 2 W 0 aa N+½d W 1 X N+½d ba W 1 A Relaxation back to equilibrium can occur through: Zero-quantum transitions (W 0) Single quantum transitions (W 1) Double quantum transitions (W 2)

Nuclear Overhauser Effect (NOE) Intensity of NOE “builds-up” as a function of the mixing time (tm) ab W 1 A N-½d W 1 X bb N-½d W 2 W 0 aa N+½d W 1 X N+½d ba W 1 A Solomon Equation: Steady-state NOE enhancement at spin A is a function of all the relaxation pathways If only W 1, no NOE effect at HA If W 0 is dominant, decrease in intensity at HA negative NOE If W 2 is dominate, increase in intensity at HA positive NOE For homonuclear (g. X=g. A), maximum enhancement is ~ 50% For heteronuclear (g. X=g. A), maximum enhancement is ~50%(g. X/g. A)

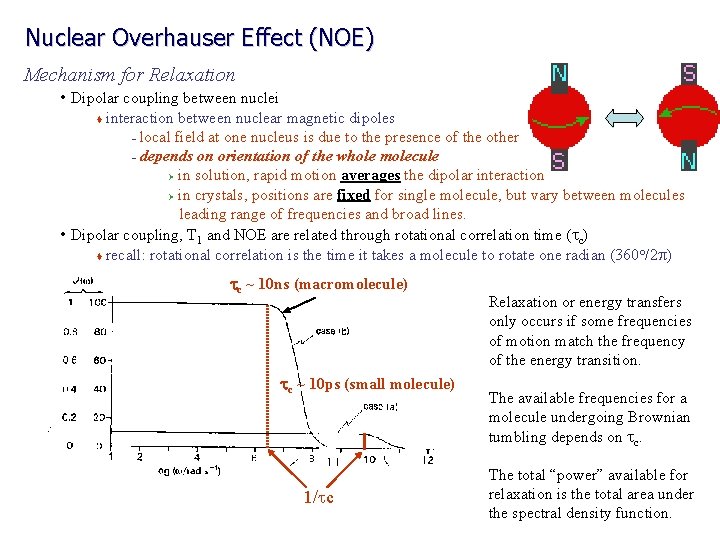
Nuclear Overhauser Effect (NOE) Mechanism for Relaxation • Dipolar coupling between nuclei interaction between nuclear magnetic dipoles – local field at one nucleus is due to the presence of the other – depends on orientation of the whole molecule Ø in solution, rapid motion averages the dipolar interaction Ø in crystals, positions are fixed for single molecule, but vary between molecules leading range of frequencies and broad lines. • Dipolar coupling, T 1 and NOE are related through rotational correlation time (tc) o t recall: rotational correlation is the time it takes a molecule to rotate one radian (360 /2 p) t tc ~ 10 ns (macromolecule) tc ~ 10 ps (small molecule) 1/tc Relaxation or energy transfers only occurs if some frequencies of motion match the frequency of the energy transition. The available frequencies for a molecule undergoing Brownian tumbling depends on tc. The total “power” available for relaxation is the total area under the spectral density function.

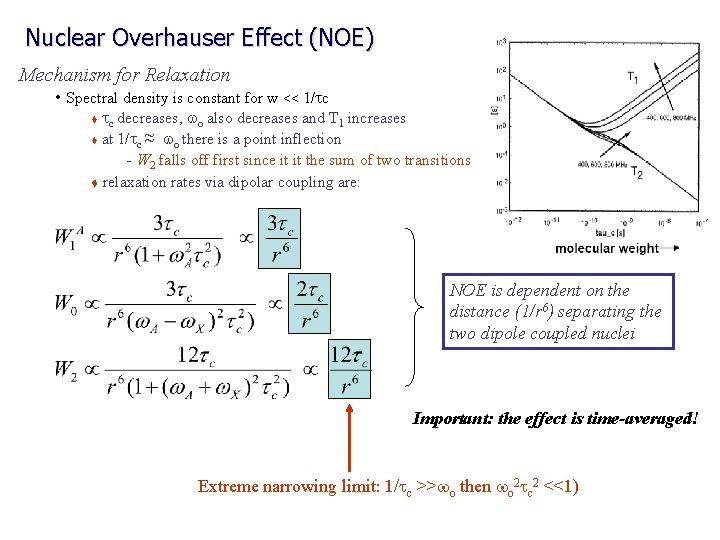
Nuclear Overhauser Effect (NOE) Mechanism for Relaxation • Spectral density is constant for w << 1/tc tc decreases, wo also decreases and T 1 increases t at 1/tc ≈ wo there is a point inflection – W 2 falls off first since it it the sum of two transitions t relaxation rates via dipolar coupling are: t NOE is dependent on the distance (1/r 6) separating the two dipole coupled nuclei Important: the effect is time-averaged! Extreme narrowing limit: 1/tc >>wo then wo 2 tc 2 <<1)

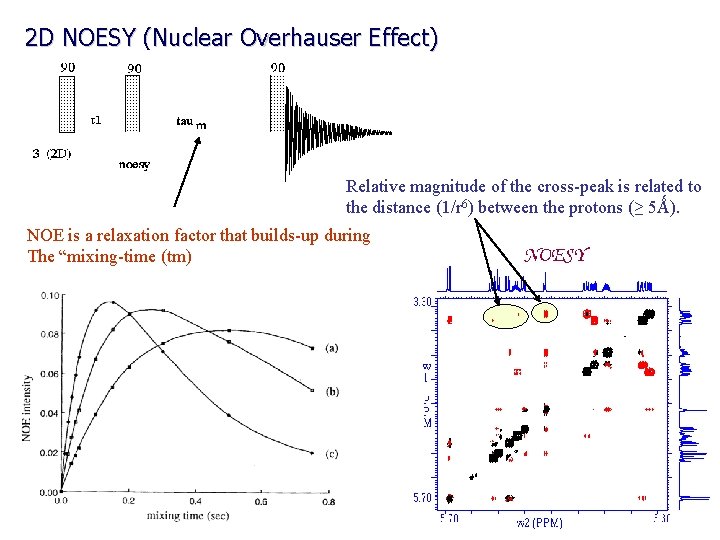
2 D NOESY (Nuclear Overhauser Effect) Relative magnitude of the cross-peak is related to the distance (1/r 6) between the protons (≥ 5Ǻ). NOE is a relaxation factor that builds-up during The “mixing-time (tm)

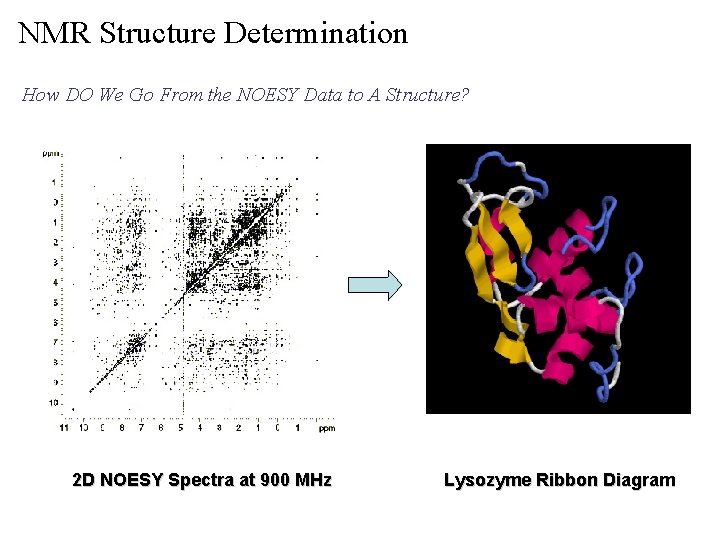
NMR Structure Determination How DO We Go From the NOESY Data to A Structure? 2 D NOESY Spectra at 900 MHz Lysozyme Ribbon Diagram

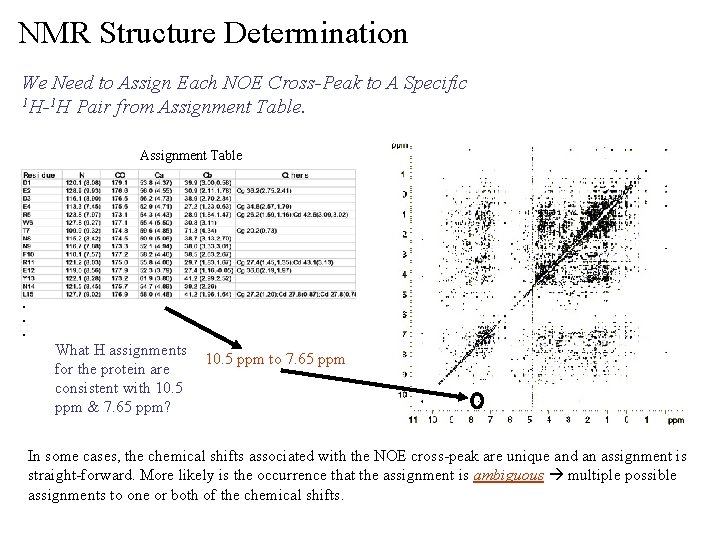
NMR Structure Determination We Need to Assign Each NOE Cross-Peak to A Specific 1 H-1 H Pair from Assignment Table . . . What H assignments for the protein are consistent with 10. 5 ppm & 7. 65 ppm? 10. 5 ppm to 7. 65 ppm In some cases, the chemical shifts associated with the NOE cross-peak are unique and an assignment is straight-forward. More likely is the occurrence that the assignment is ambiguous multiple possible assignments to one or both of the chemical shifts.

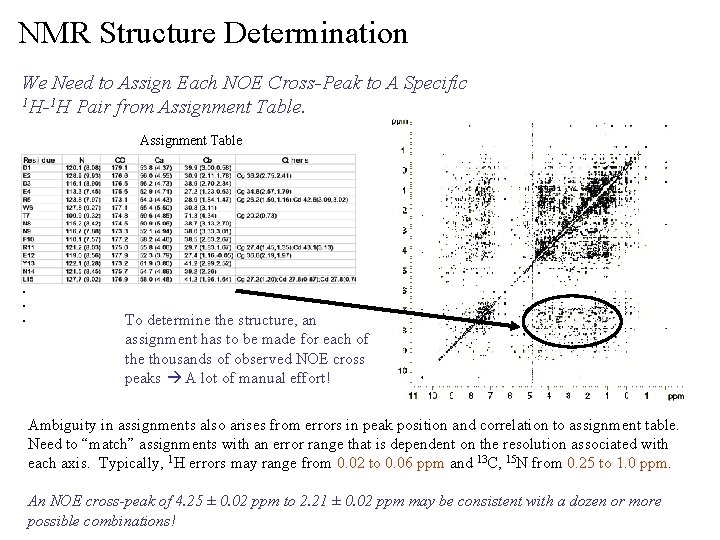
NMR Structure Determination We Need to Assign Each NOE Cross-Peak to A Specific 1 H-1 H Pair from Assignment Table . . . To determine the structure, an assignment has to be made for each of the thousands of observed NOE cross peaks A lot of manual effort! Ambiguity in assignments also arises from errors in peak position and correlation to assignment table. Need to “match” assignments with an error range that is dependent on the resolution associated with each axis. Typically, 1 H errors may range from 0. 02 to 0. 06 ppm and 13 C, 15 N from 0. 25 to 1. 0 ppm. An NOE cross-peak of 4. 25 ± 0. 02 ppm to 2. 21 ± 0. 02 ppm may be consistent with a dozen or more possible combinations!

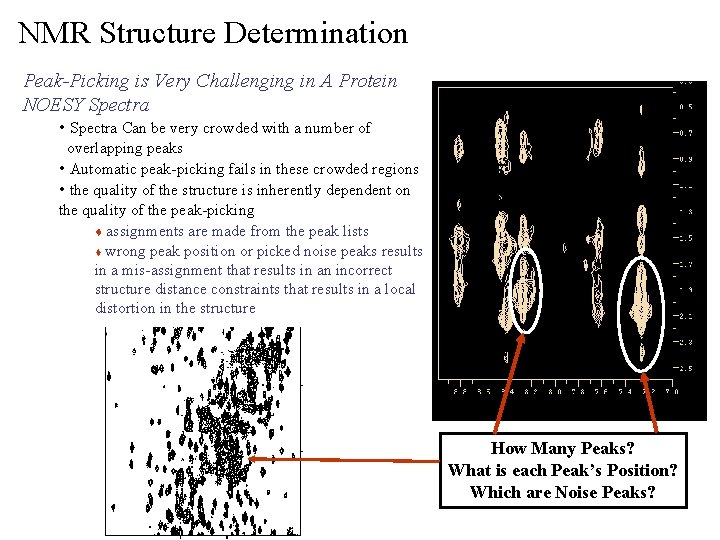
NMR Structure Determination Peak-Picking is Very Challenging in A Protein NOESY Spectra • Spectra Can be very crowded with a number of overlapping peaks • Automatic peak-picking fails in these crowded regions • the quality of the structure is inherently dependent on the quality of the peak-picking t assignments are made from the peak lists t wrong peak position or picked noise peaks results in a mis-assignment that results in an incorrect structure distance constraints that results in a local distortion in the structure How Many Peaks? What is each Peak’s Position? Which are Noise Peaks?

NMR Structure Determination How Do We Resolve These Peak-Picking and Ambiguity Issues? • Spread out the data into 3 D and 4 D symmetry can help remove ambiguities • Use 1 H-15 N and 1 H-13 C connections • Iterative analysis of the NOE data t use structure and distance filter to remove ambiguities t

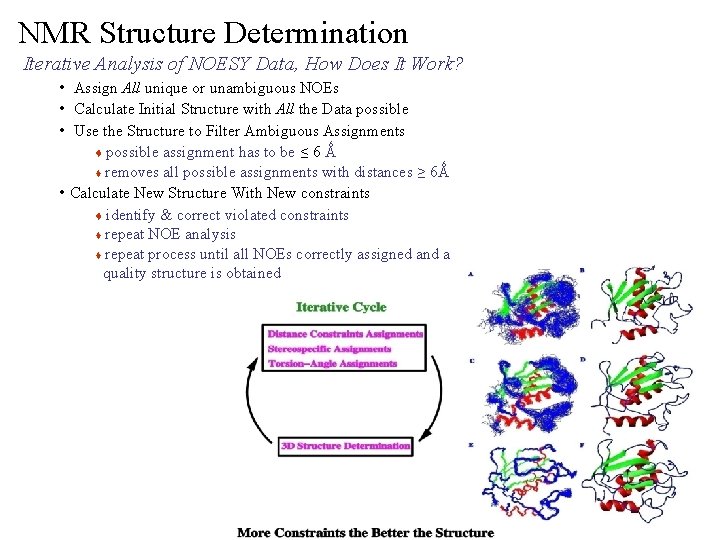
NMR Structure Determination Iterative Analysis of NOESY Data, How Does It Work? • Assign All unique or unambiguous NOEs • Calculate Initial Structure with All the Data possible • Use the Structure to Filter Ambiguous Assignments possible assignment has to be ≤ 6 Ǻ t removes all possible assignments with distances ≥ 6Ǻ • Calculate New Structure With New constraints t identify & correct violated constraints t repeat NOE analysis t repeat process until all NOEs correctly assigned and a quality structure is obtained t

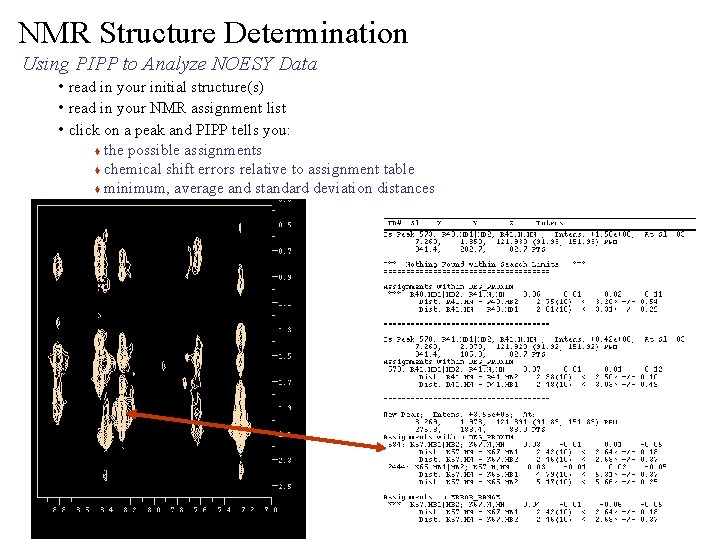
NMR Structure Determination Using PIPP to Analyze NOESY Data • read in your initial structure(s) • read in your NMR assignment list • click on a peak and PIPP tells you: the possible assignments t chemical shift errors relative to assignment table t minimum, average and standard deviation distances t

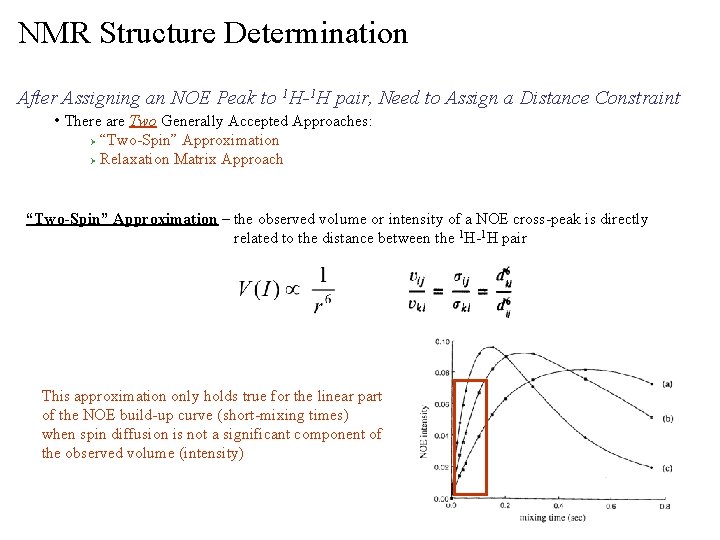
NMR Structure Determination After Assigning an NOE Peak to 1 H-1 H pair, Need to Assign a Distance Constraint • There are Two Generally Accepted Approaches: “Two-Spin” Approximation Ø Relaxation Matrix Approach Ø “Two-Spin” Approximation – the observed volume or intensity of a NOE cross-peak is directly related to the distance between the 1 H-1 H pair This approximation only holds true for the linear part of the NOE build-up curve (short-mixing times) when spin diffusion is not a significant component of the observed volume (intensity)

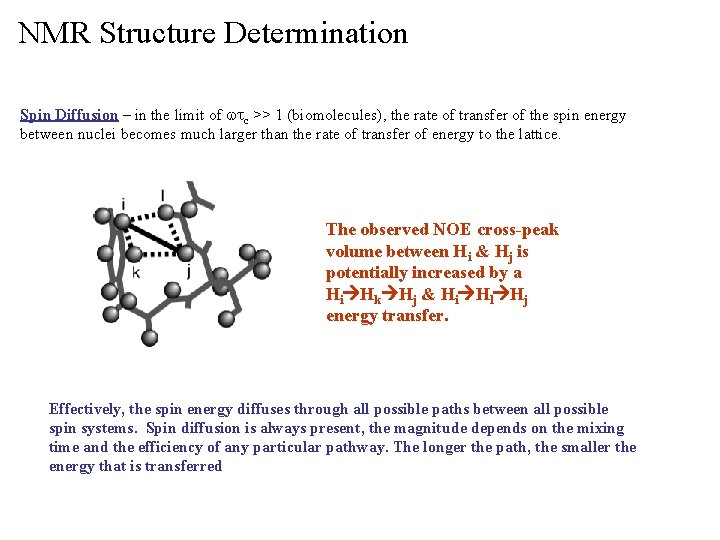
NMR Structure Determination Spin Diffusion – in the limit of wtc >> 1 (biomolecules), the rate of transfer of the spin energy between nuclei becomes much larger than the rate of transfer of energy to the lattice. The observed NOE cross-peak volume between Hi & Hj is potentially increased by a Hi Hk Hj & Hi Hl Hj energy transfer. Effectively, the spin energy diffuses through all possible paths between all possible spin systems. Spin diffusion is always present, the magnitude depends on the mixing time and the efficiency of any particular pathway. The longer the path, the smaller the energy that is transferred

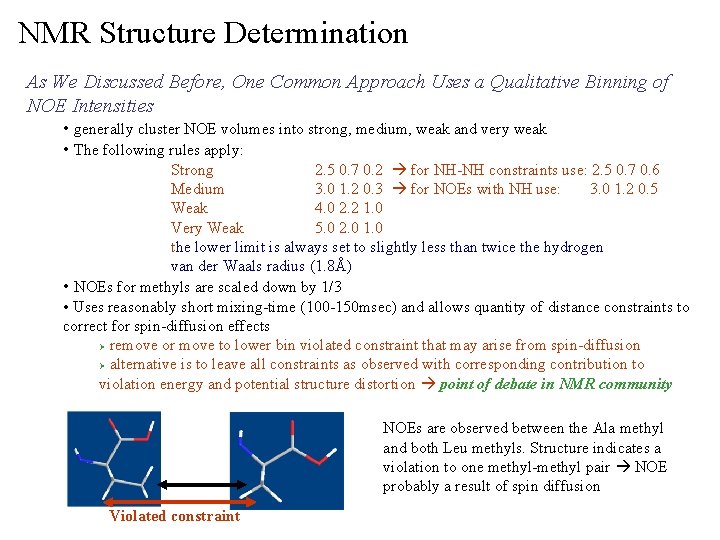
NMR Structure Determination As We Discussed Before, One Common Approach Uses a Qualitative Binning of NOE Intensities • generally cluster NOE volumes into strong, medium, weak and very weak • The following rules apply: Strong 2. 5 0. 7 0. 2 for NH-NH constraints use: 2. 5 0. 7 0. 6 Medium 3. 0 1. 2 0. 3 for NOEs with NH use: 3. 0 1. 2 0. 5 Weak 4. 0 2. 2 1. 0 Very Weak 5. 0 2. 0 1. 0 the lower limit is always set to slightly less than twice the hydrogen van der Waals radius (1. 8Å) • NOEs for methyls are scaled down by 1/3 • Uses reasonably short mixing-time (100 -150 msec) and allows quantity of distance constraints to correct for spin-diffusion effects Ø remove or move to lower bin violated constraint that may arise from spin-diffusion Ø alternative is to leave all constraints as observed with corresponding contribution to violation energy and potential structure distortion point of debate in NMR community NOEs are observed between the Ala methyl and both Leu methyls. Structure indicates a violation to one methyl-methyl pair NOE probably a result of spin diffusion Violated constraint

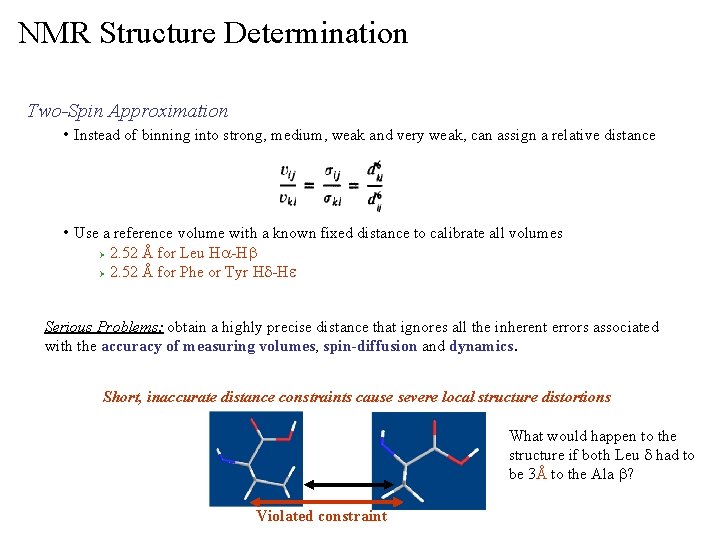
NMR Structure Determination Two-Spin Approximation • Instead of binning into strong, medium, weak and very weak, can assign a relative distance • Use a reference volume with a known fixed distance to calibrate all volumes 2. 52 Å for Leu Ha-Hb Ø 2. 52 Å for Phe or Tyr Hd-He Ø Serious Problems: obtain a highly precise distance that ignores all the inherent errors associated with the accuracy of measuring volumes, spin-diffusion and dynamics. Short, inaccurate distance constraints cause severe local structure distortions What would happen to the structure if both Leu d had to be 3Å to the Ala b? Violated constraint

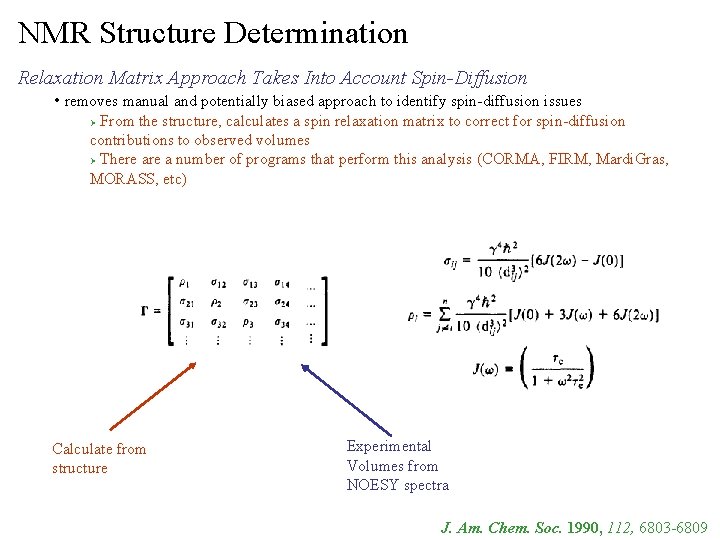
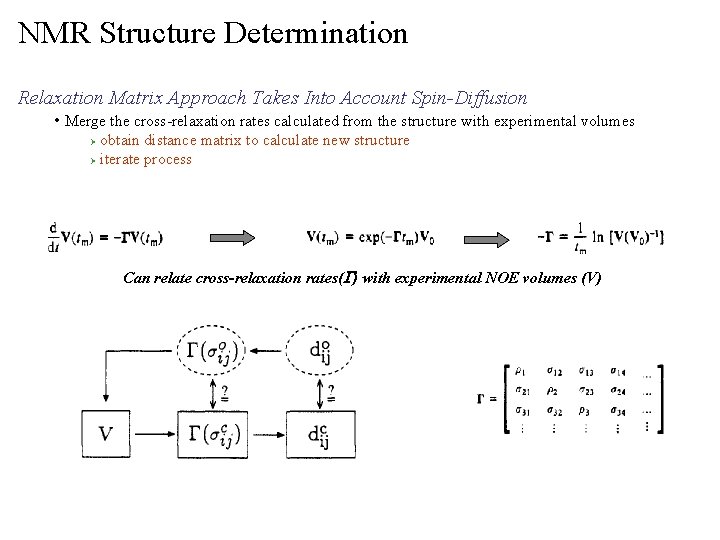
NMR Structure Determination Relaxation Matrix Approach Takes Into Account Spin-Diffusion • removes manual and potentially biased approach to identify spin-diffusion issues From the structure, calculates a spin relaxation matrix to correct for spin-diffusion contributions to observed volumes Ø There a number of programs that perform this analysis (CORMA, FIRM, Mardi. Gras, MORASS, etc) Ø Calculate from structure Experimental Volumes from NOESY spectra J. Am. Chem. Soc. 1990, 112, 6803 -6809

NMR Structure Determination Relaxation Matrix Approach Takes Into Account Spin-Diffusion • Merge the cross-relaxation rates calculated from the structure with experimental volumes obtain distance matrix to calculate new structure Ø iterate process Ø Can relate cross-relaxation rates(G) with experimental NOE volumes (V)

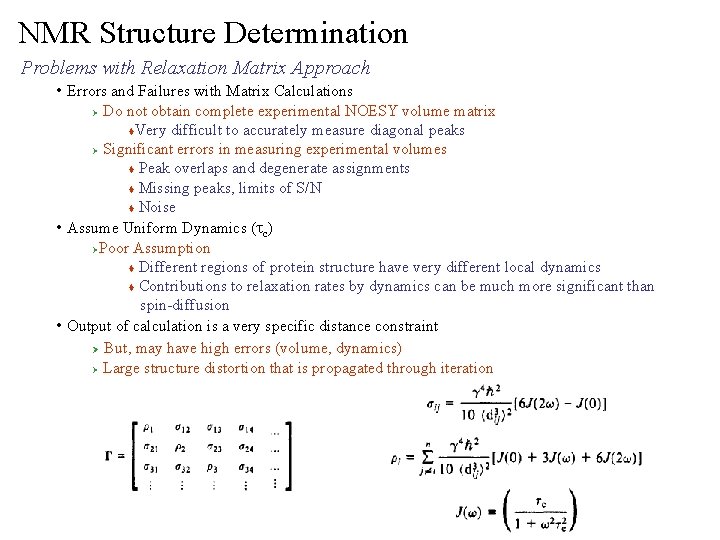
NMR Structure Determination Problems with Relaxation Matrix Approach • Errors and Failures with Matrix Calculations Do not obtain complete experimental NOESY volume matrix t. Very difficult to accurately measure diagonal peaks Ø Significant errors in measuring experimental volumes t Peak overlaps and degenerate assignments t Missing peaks, limits of S/N t Noise • Assume Uniform Dynamics (tc) ØPoor Assumption t Different regions of protein structure have very different local dynamics t Contributions to relaxation rates by dynamics can be much more significant than spin-diffusion • Output of calculation is a very specific distance constraint Ø But, may have high errors (volume, dynamics) Ø Large structure distortion that is propagated through iteration Ø

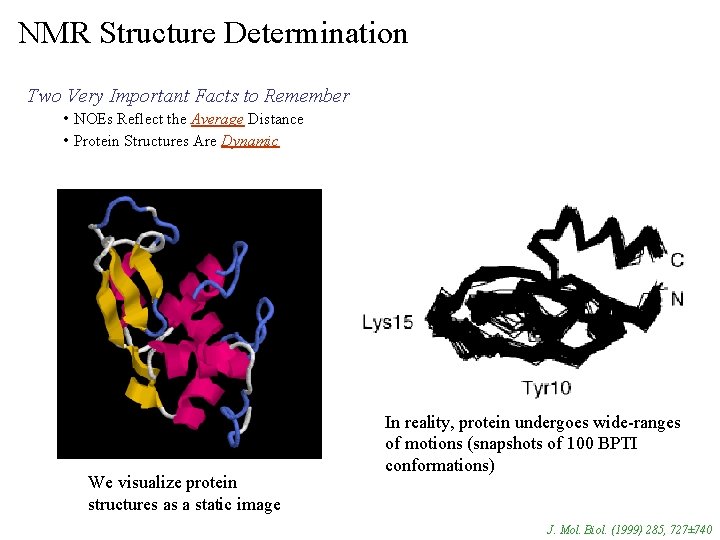
NMR Structure Determination Two Very Important Facts to Remember • NOEs Reflect the Average Distance • Protein Structures Are Dynamic We visualize protein structures as a static image In reality, protein undergoes wide-ranges of motions (snapshots of 100 BPTI conformations) J. Mol. Biol. (1999) 285, 727± 740

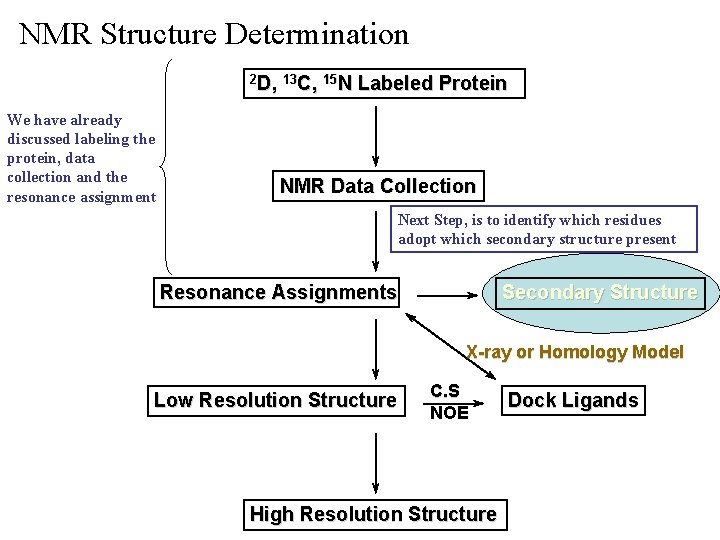
NMR Structure Determination 2 D, 13 C, 15 N We have already discussed labeling the protein, data collection and the resonance assignment Labeled Protein NMR Data Collection Next Step, is to identify which residues adopt which secondary structure present Resonance Assignments Secondary Structure X-ray or Homology Model Low Resolution Structure C. S NOE High Resolution Structure Dock Ligands

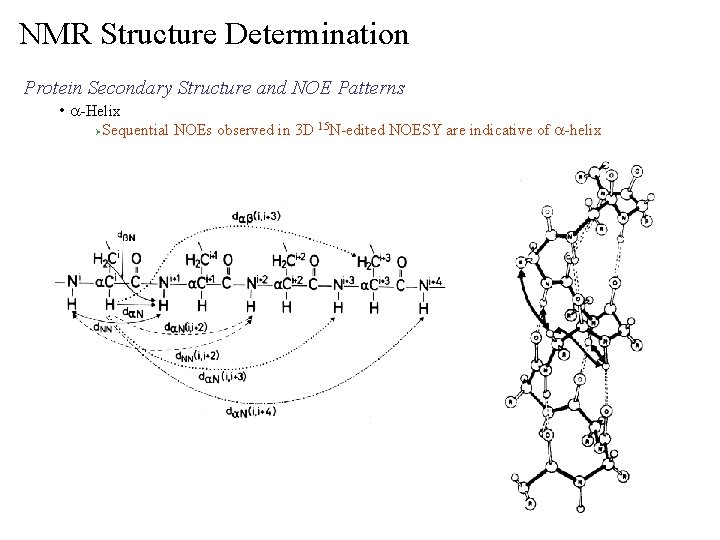
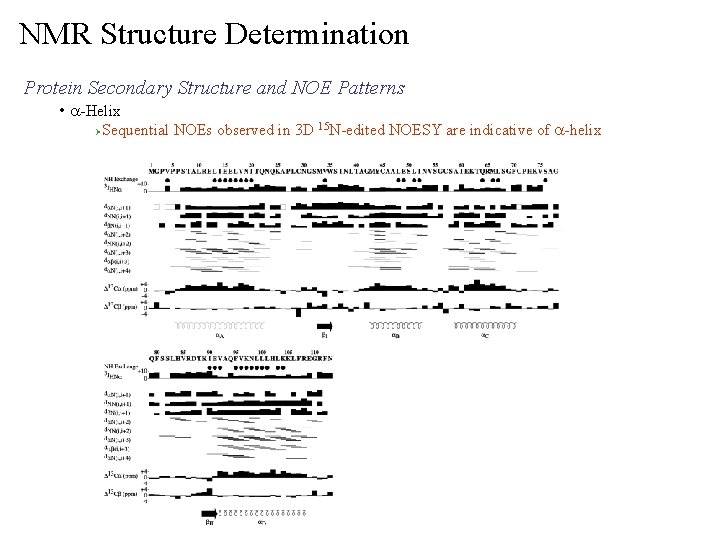
NMR Structure Determination Protein Secondary Structure and NOE Patterns • a-Helix Ø Sequential NOEs observed in 3 D 15 N-edited NOESY are indicative of a-helix

NMR Structure Determination Protein Secondary Structure and NOE Patterns • a-Helix Ø Sequential NOEs observed in 3 D 15 N-edited NOESY are indicative of a-helix

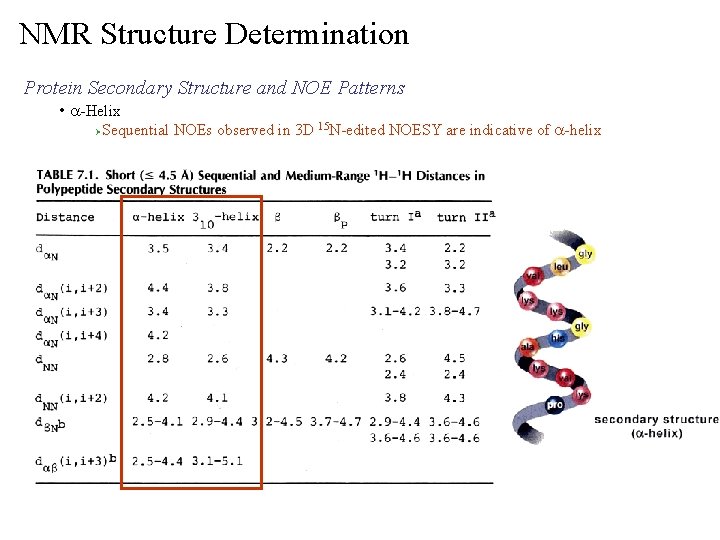
NMR Structure Determination Protein Secondary Structure and NOE Patterns • a-Helix Ø Sequential NOEs observed in 3 D 15 N-edited NOESY are indicative of a-helix

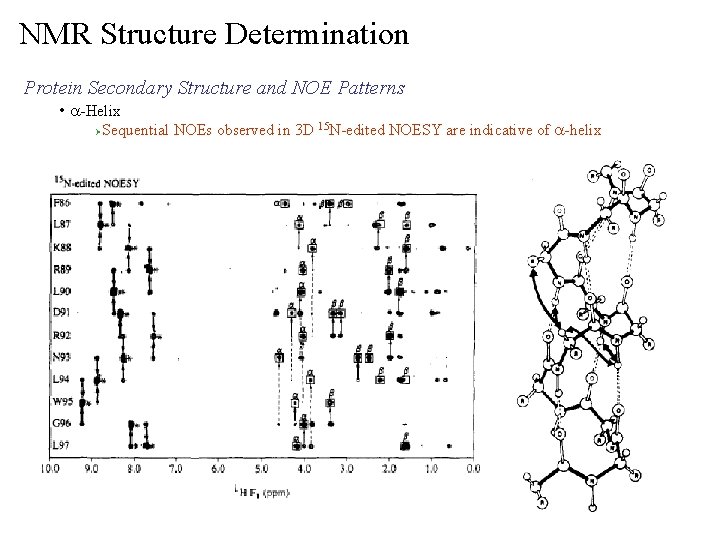
NMR Structure Determination Protein Secondary Structure and NOE Patterns • a-Helix Ø Sequential NOEs observed in 3 D 15 N-edited NOESY are indicative of a-helix

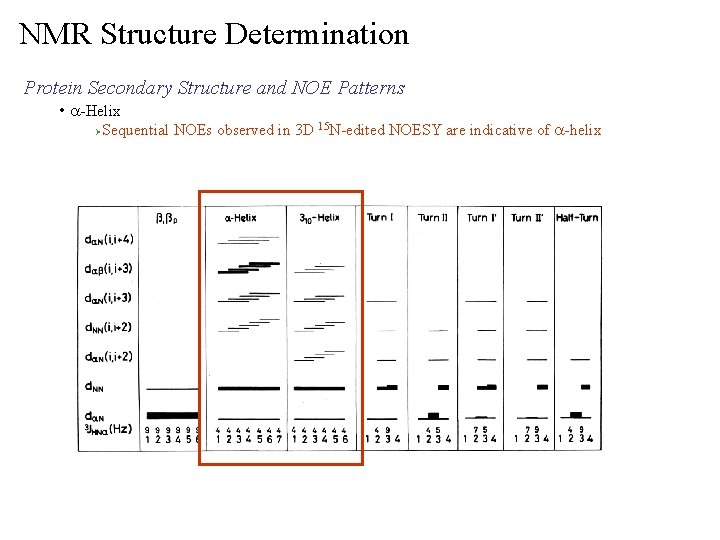
NMR Structure Determination Protein Secondary Structure and NOE Patterns • a-Helix Ø Sequential NOEs observed in 3 D 15 N-edited NOESY are indicative of a-helix

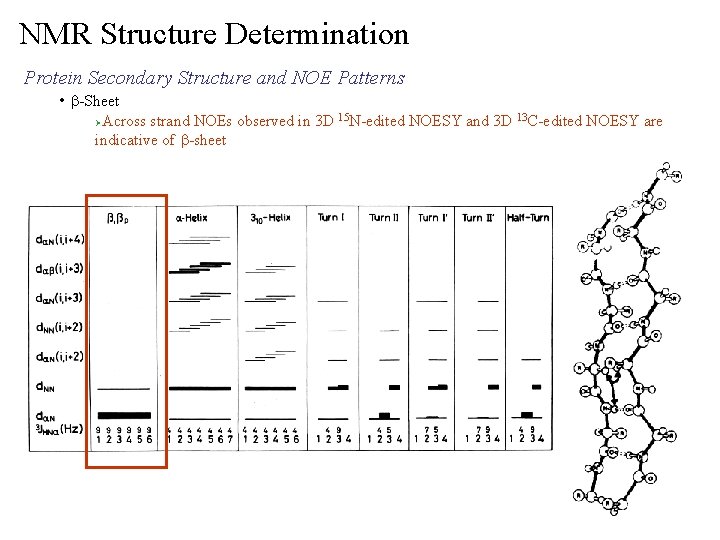
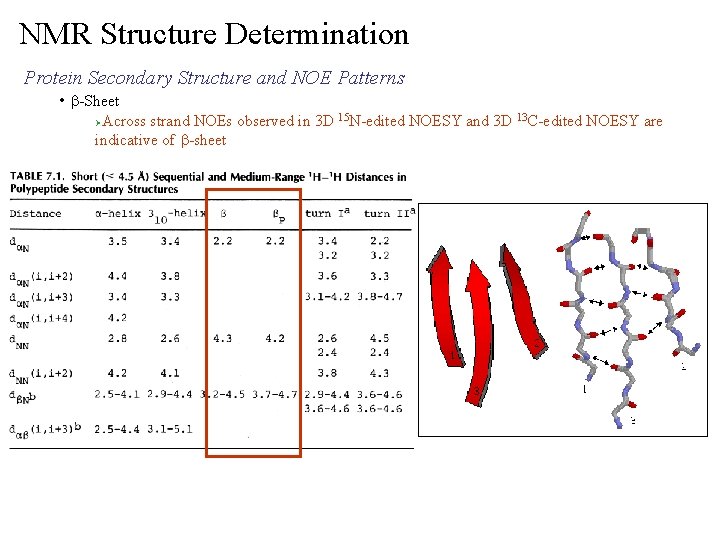
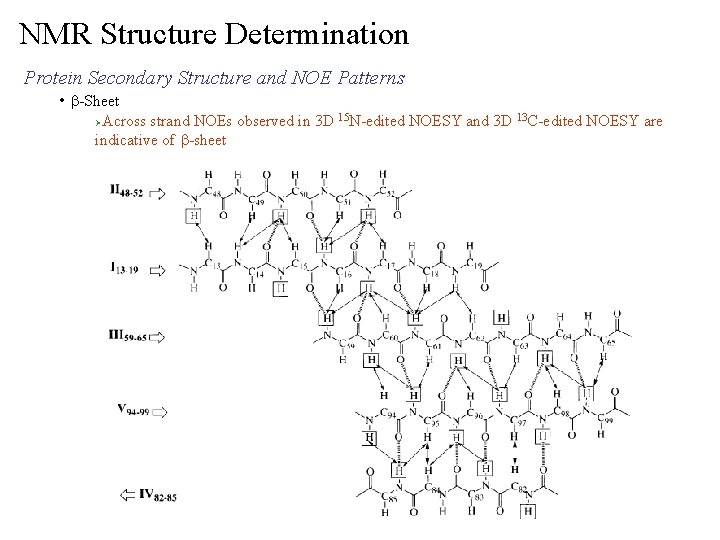
NMR Structure Determination Protein Secondary Structure and NOE Patterns • b-Sheet Across strand NOEs observed in 3 D 15 N-edited NOESY and 3 D 13 C-edited NOESY are indicative of b-sheet Ø

NMR Structure Determination Protein Secondary Structure and NOE Patterns • b-Sheet Across strand NOEs observed in 3 D 15 N-edited NOESY and 3 D 13 C-edited NOESY are indicative of b-sheet Ø

NMR Structure Determination Protein Secondary Structure and NOE Patterns • b-Sheet Across strand NOEs observed in 3 D 15 N-edited NOESY and 3 D 13 C-edited NOESY are indicative of b-sheet Ø

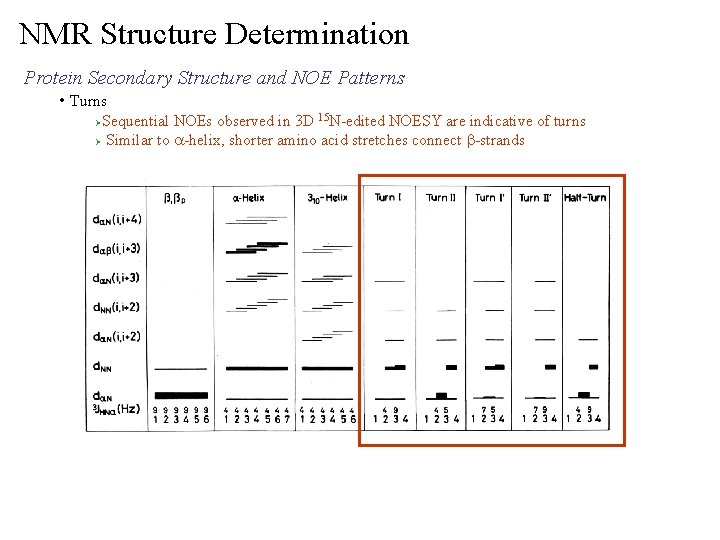
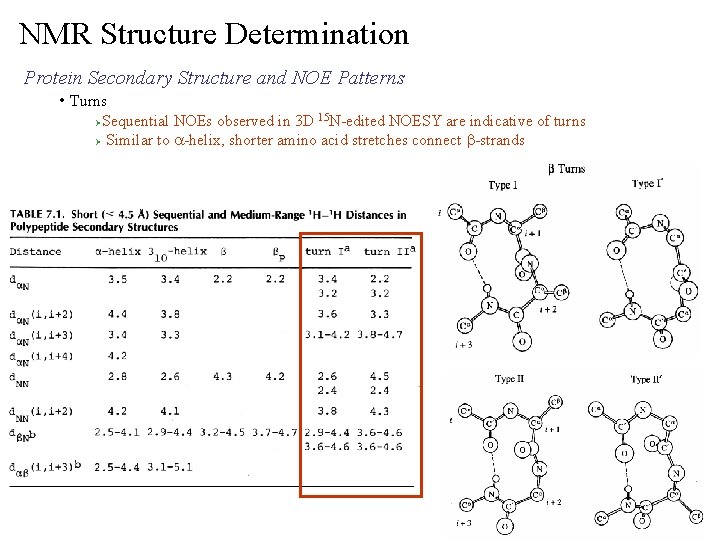
NMR Structure Determination Protein Secondary Structure and NOE Patterns • Turns Sequential NOEs observed in 3 D 15 N-edited NOESY are indicative of turns Ø Similar to a-helix, shorter amino acid stretches connect b-strands Ø

NMR Structure Determination Protein Secondary Structure and NOE Patterns • Turns Sequential NOEs observed in 3 D 15 N-edited NOESY are indicative of turns Ø Similar to a-helix, shorter amino acid stretches connect b-strands Ø

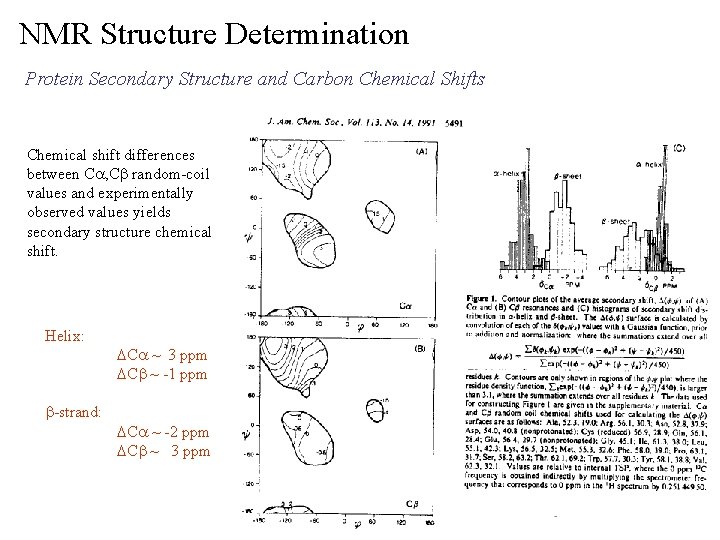
NMR Structure Determination Protein Secondary Structure and Carbon Chemical Shifts Chemical shift differences between Ca, Cb random-coil values and experimentally observed values yields secondary structure chemical shift. Helix: b-strand: DCa ~ 3 ppm DCb ~ -1 ppm DCa ~ -2 ppm DCb ~ 3 ppm

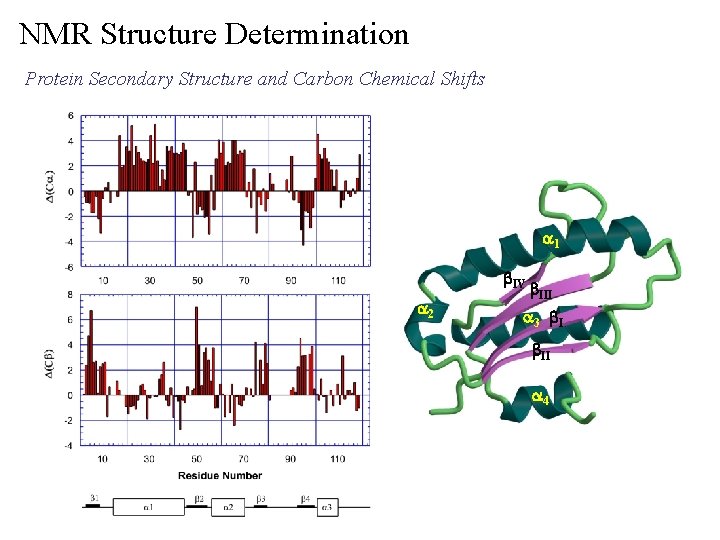
NMR Structure Determination Protein Secondary Structure and Carbon Chemical Shifts a 1 b. IV a 2 b. III a 3 b. II a 4

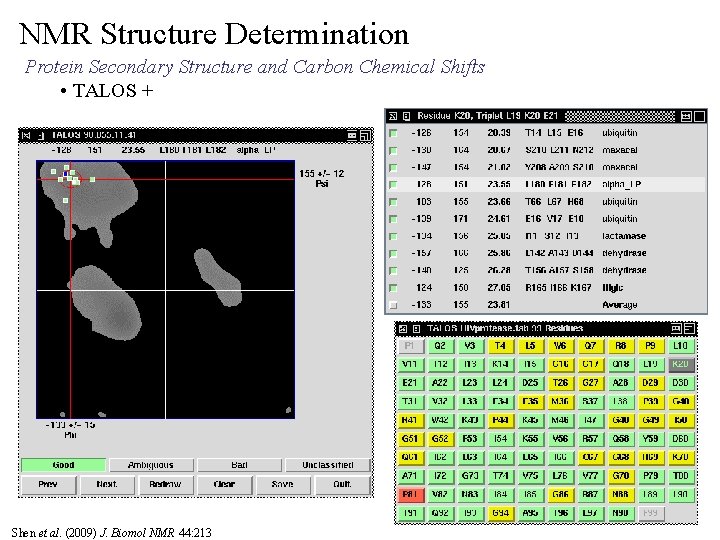
NMR Structure Determination Protein Secondary Structure and Carbon Chemical Shifts • TALOS + Shen et al. (2009) J. Biomol NMR 44: 213

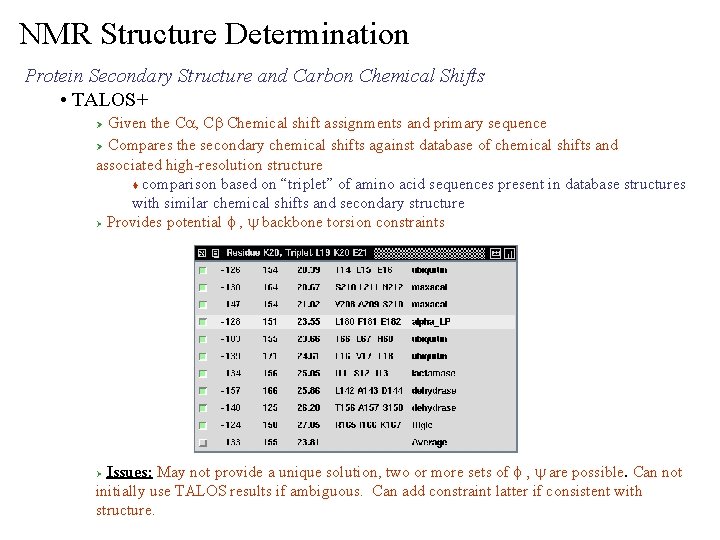
NMR Structure Determination Protein Secondary Structure and Carbon Chemical Shifts • TALOS+ Given the Ca, Cb Chemical shift assignments and primary sequence Ø Compares the secondary chemical shifts against database of chemical shifts and associated high-resolution structure t comparison based on “triplet” of amino acid sequences present in database structures with similar chemical shifts and secondary structure Ø Provides potential f , y backbone torsion constraints Ø Issues: May not provide a unique solution, two or more sets of f , y are possible. Can not initially use TALOS results if ambiguous. Can add constraint latter if consistent with structure. Ø

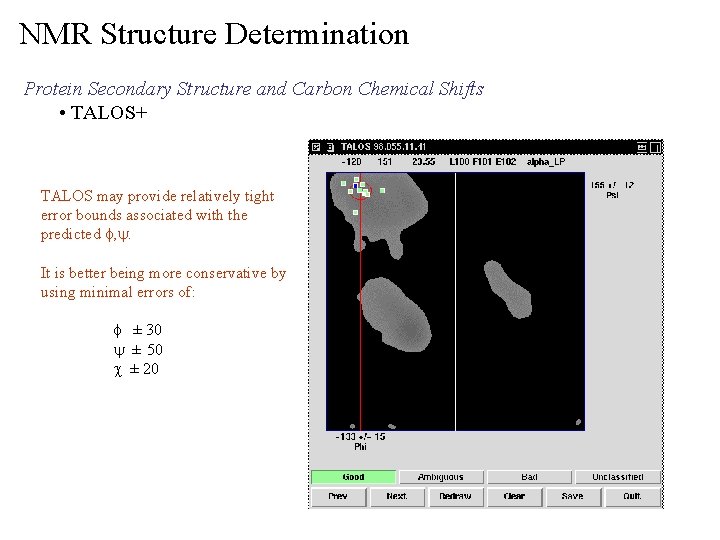
NMR Structure Determination Protein Secondary Structure and Carbon Chemical Shifts • TALOS+ TALOS may provide relatively tight error bounds associated with the predicted f, y. It is better being more conservative by using minimal errors of: f ± 30 y ± 50 c ± 20

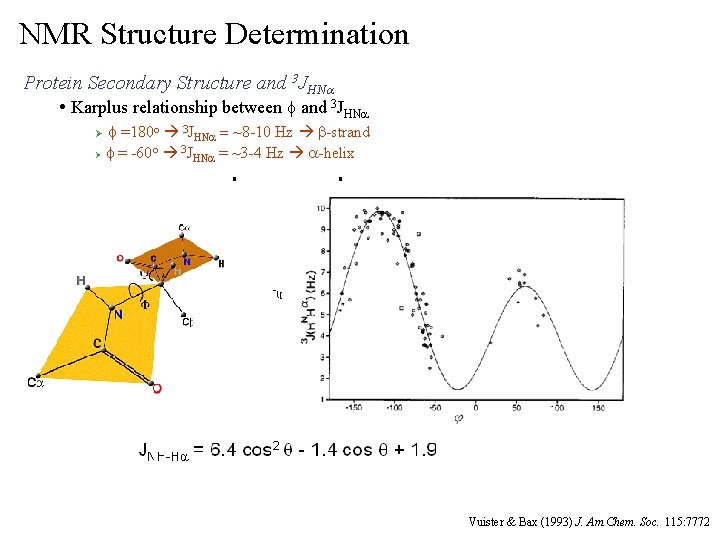
NMR Structure Determination Protein Secondary Structure and 3 JHNa • Karplus relationship between f and 3 JHNa f =180 o 3 JHNa = ~8 -10 Hz b-strand o 3 Ø f = -60 JHNa = ~3 -4 Hz a-helix Ø Vuister & Bax (1993) J. Am. Chem. Soc. 115: 7772

NMR Structure Determination Protein Secondary Structure and 3 JHNa • Karplus relationship between f and 3 JHNa Ø Ø Measure 3 JHNa for a protein using HNHA Ratio of cross-peak to diagonal intensity yields coupling constant t Common approach to measure coupling constants in complex protein NMR spectra J. Am. Chem. Soc. 1993, 115, 7772 -7777

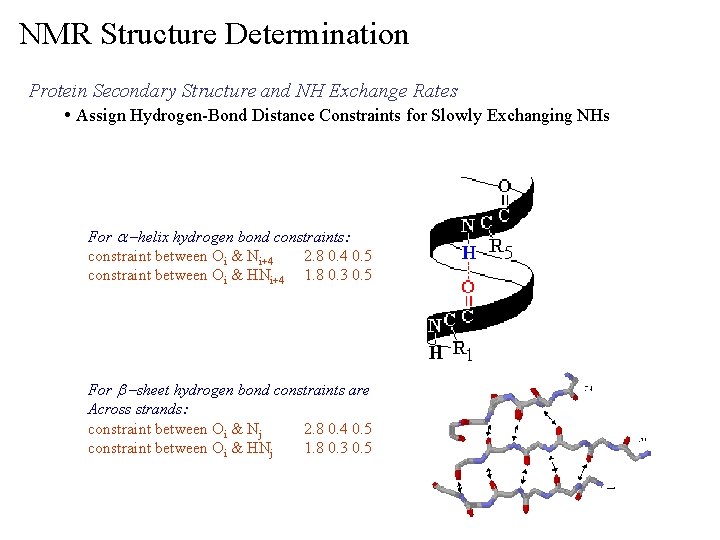
NMR Structure Determination Protein Secondary Structure and NH Exchange Rates • Relatively Slower Exchanging NHs are involved in Hydrogen Bonds or Buried Ø Hydrogen Bonds are an important component of secondary structures NH Exchange Rate

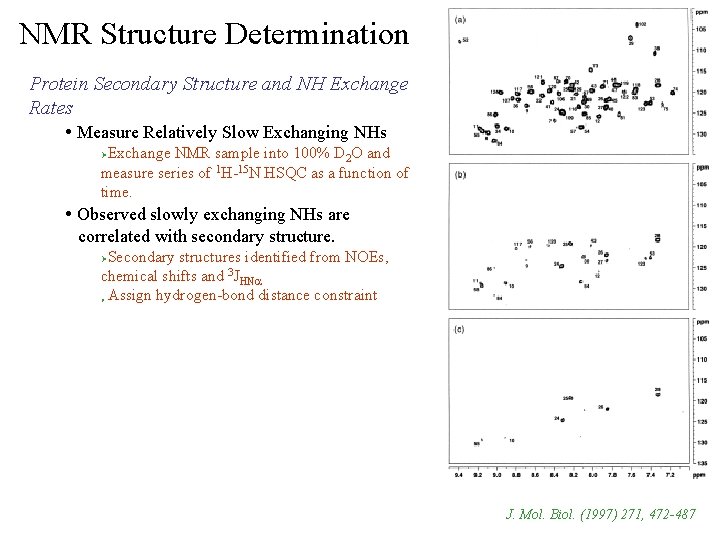
NMR Structure Determination Protein Secondary Structure and NH Exchange Rates • Measure Relatively Slow Exchanging NHs Exchange NMR sample into 100% D 2 O and measure series of 1 H-15 N HSQC as a function of time. Ø • Observed slowly exchanging NHs are correlated with secondary structure. Secondary structures identified from NOEs, chemical shifts and 3 JHNa Assign hydrogen-bond distance constraint Ø Ø J. Mol. Biol. (1997) 271, 472 -487

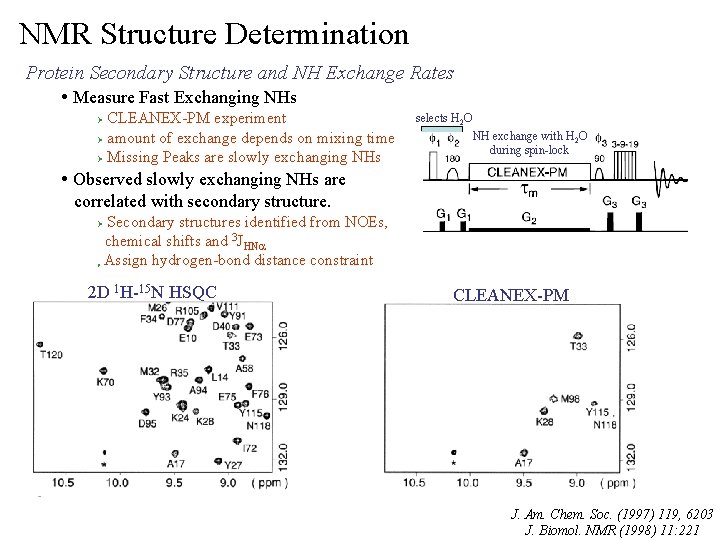
NMR Structure Determination Protein Secondary Structure and NH Exchange Rates • Measure Fast Exchanging NHs CLEANEX-PM experiment Ø amount of exchange depends on mixing time Ø Missing Peaks are slowly exchanging NHs Ø selects H 2 O NH exchange with H 2 O during spin-lock • Observed slowly exchanging NHs are correlated with secondary structure. Ø Ø Secondary structures identified from NOEs, chemical shifts and 3 JHNa Assign hydrogen-bond distance constraint 2 D 1 H-15 N HSQC CLEANEX-PM J. Am. Chem. Soc. (1997) 119, 6203 J. Biomol. NMR (1998) 11: 221

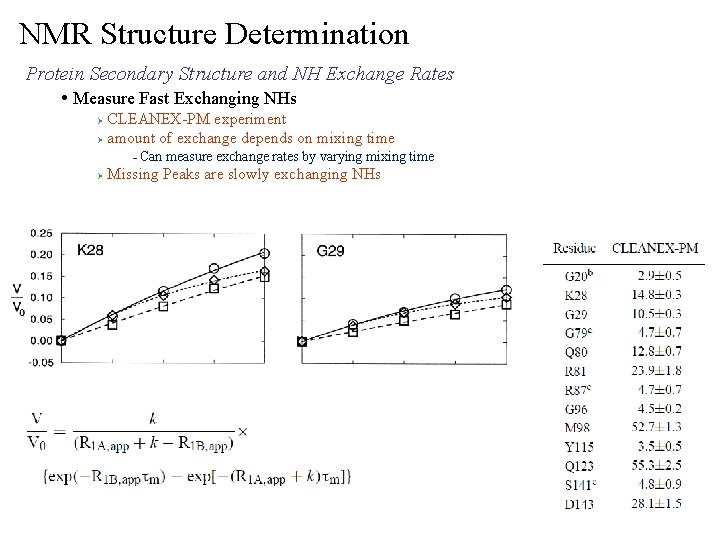
NMR Structure Determination Protein Secondary Structure and NH Exchange Rates • Measure Fast Exchanging NHs CLEANEX-PM experiment Ø amount of exchange depends on mixing time Ø – Ø Can measure exchange rates by varying mixing time Missing Peaks are slowly exchanging NHs

NMR Structure Determination Protein Secondary Structure and NH Exchange Rates • Assign Hydrogen-Bond Distance Constraints for Slowly Exchanging NHs For a –helix hydrogen bond constraints: constraint between Oi & Ni+4 2. 8 0. 4 0. 5 constraint between Oi & HNi+4 1. 8 0. 3 0. 5 For b –sheet hydrogen bond constraints are Across strands: constraint between Oi & Nj 2. 8 0. 4 0. 5 constraint between Oi & HNj 1. 8 0. 3 0. 5

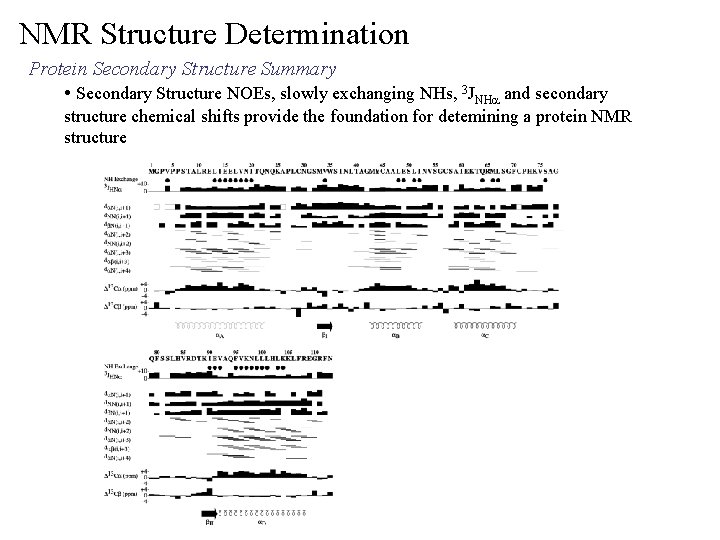
NMR Structure Determination Protein Secondary Structure Summary • Secondary Structure NOEs, slowly exchanging NHs, 3 JNHa and secondary structure chemical shifts provide the foundation for detemining a protein NMR structure

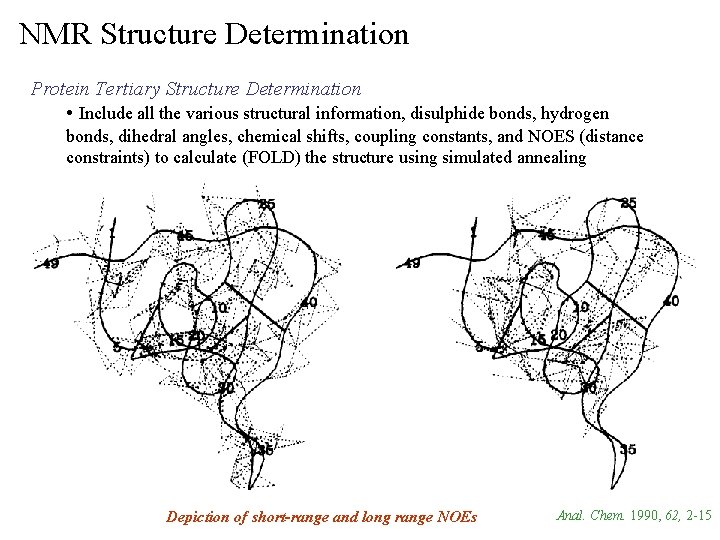
NMR Structure Determination Protein Tertiary Structure Determination • Include all the various structural information, disulphide bonds, hydrogen bonds, dihedral angles, chemical shifts, coupling constants, and NOES (distance constraints) to calculate (FOLD) the structure using simulated annealing Depiction of short-range and long range NOEs Anal. Chem. 1990, 62, 2 -15

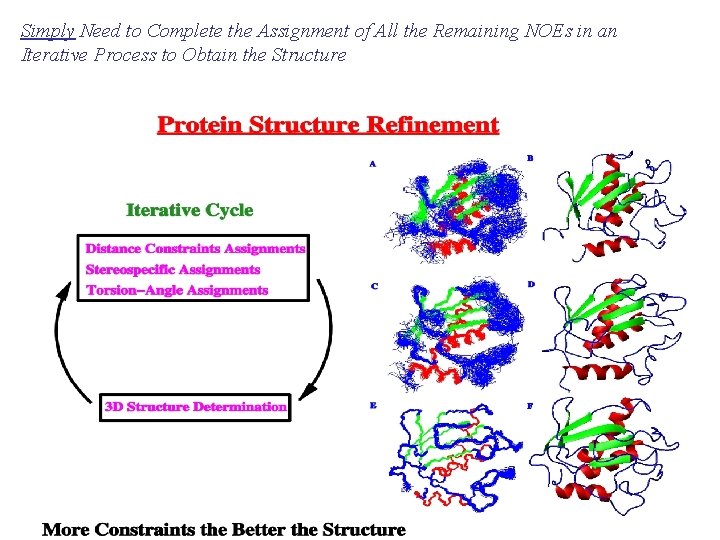
Simply Need to Complete the Assignment of All the Remaining NOEs in an Iterative Process to Obtain the Structure

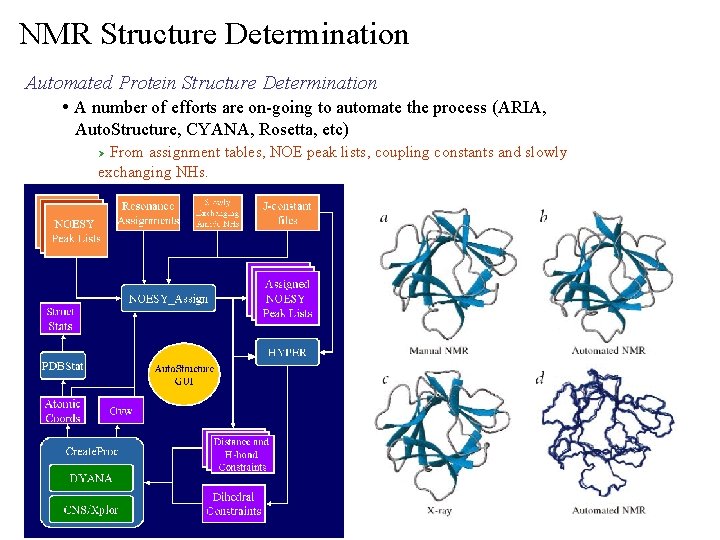
NMR Structure Determination Automated Protein Structure Determination • A number of efforts are on-going to automate the process (ARIA, Auto. Structure, CYANA, Rosetta, etc) From assignment tables, NOE peak lists, coupling constants and slowly exchanging NHs. Ø

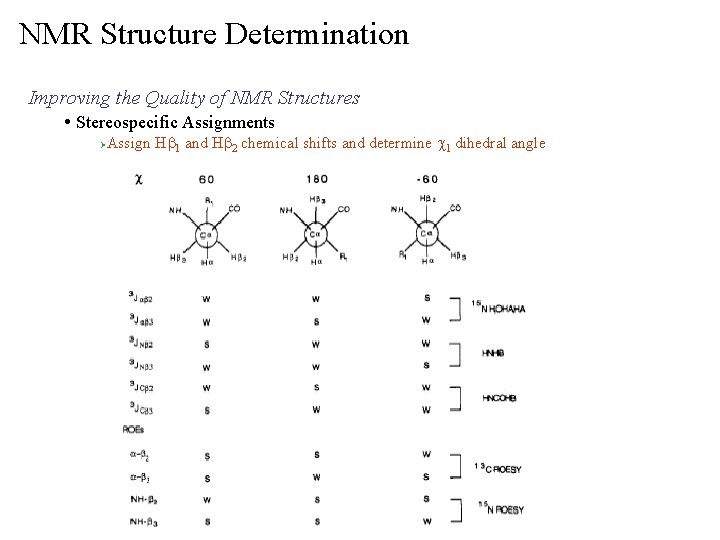
NMR Structure Determination Improving the Quality of NMR Structures • Stereospecific Assignments Ø Assign Hb 1 and Hb 2 chemical shifts and determine c 1 dihedral angle

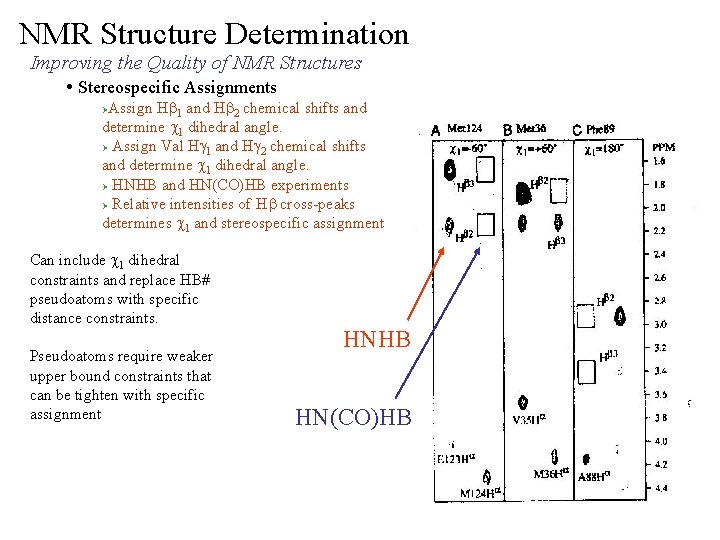
NMR Structure Determination Improving the Quality of NMR Structures • Stereospecific Assignments Assign Hb 1 and Hb 2 chemical shifts and determine c 1 dihedral angle. Ø Assign Val Hg 1 and Hg 2 chemical shifts and determine c 1 dihedral angle. Ø HNHB and HN(CO)HB experiments Ø Relative intensities of Hb cross-peaks determines c 1 and stereospecific assignment Ø Can include c 1 dihedral constraints and replace HB# pseudoatoms with specific distance constraints. Pseudoatoms require weaker upper bound constraints that can be tighten with specific assignment HNHB HN(CO)HB

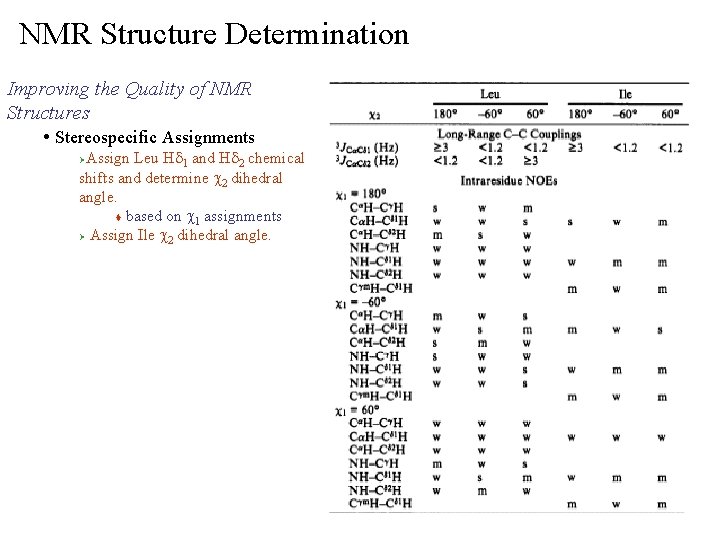
NMR Structure Determination Improving the Quality of NMR Structures • Stereospecific Assignments Assign Leu Hd 1 and Hd 2 chemical shifts and determine c 2 dihedral angle. t based on c 1 assignments Ø Assign Ile c 2 dihedral angle. Ø

NMR Structure Determination Improving the Quality of NMR Structures • Stereospecific Assignments Assign Leu Hd 1 and Hd 2 chemical shifts and determine c 2 dihedral angle. Ø Assign Ile c 2 dihedral angle. 13 C-13 C Correlation Ø Long Range Ø Relative intensities of Hd cross-peaks compared to diagonal determines 3 JCa. Cd Ø

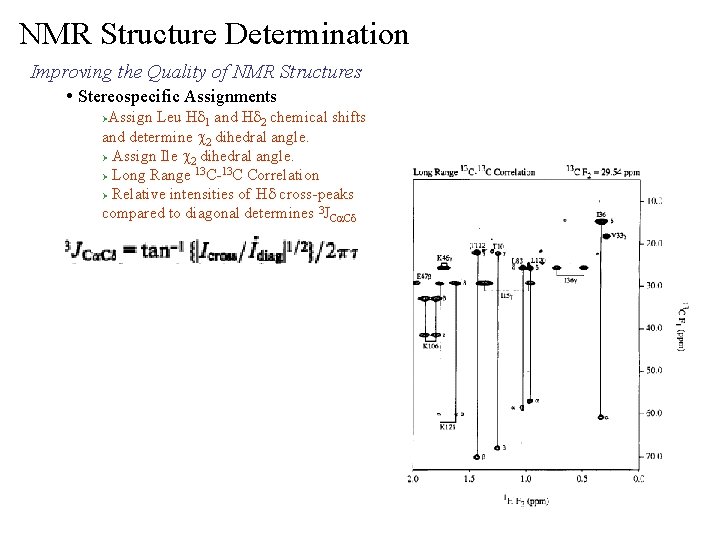
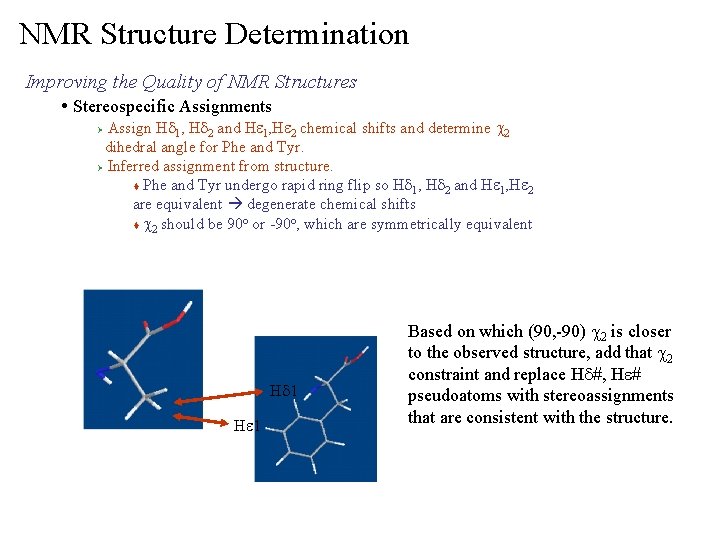
NMR Structure Determination Improving the Quality of NMR Structures • Stereospecific Assignments Assign Hd 1, Hd 2 and He 1, He 2 chemical shifts and determine c 2 dihedral angle for Phe and Tyr. Ø Inferred assignment from structure. t Phe and Tyr undergo rapid ring flip so Hd 1, Hd 2 and He 1, He 2 are equivalent degenerate chemical shifts o o t c 2 should be 90 or -90 , which are symmetrically equivalent Ø Hd 1 He 1 Based on which (90, -90) c 2 is closer to the observed structure, add that c 2 constraint and replace Hd#, He# pseudoatoms with stereoassignments that are consistent with the structure.

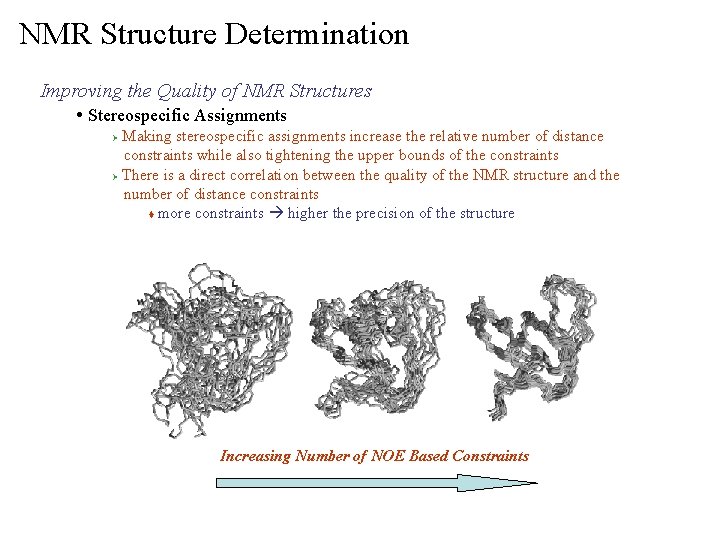
NMR Structure Determination Improving the Quality of NMR Structures • Stereospecific Assignments Making stereospecific assignments increase the relative number of distance constraints while also tightening the upper bounds of the constraints Ø There is a direct correlation between the quality of the NMR structure and the number of distance constraints t more constraints higher the precision of the structure Ø Increasing Number of NOE Based Constraints

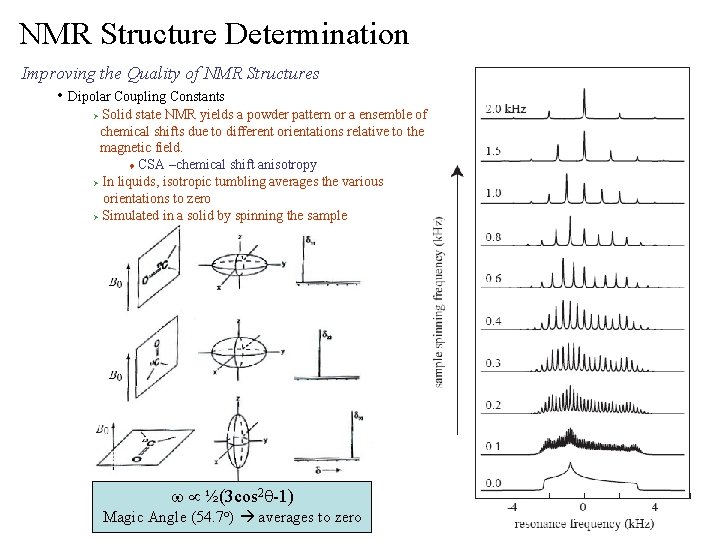
NMR Structure Determination Improving the Quality of NMR Structures • Dipolar Coupling Constants Solid state NMR yields a powder pattern or a ensemble of chemical shifts due to different orientations relative to the magnetic field. t CSA –chemical shift anisotropy Ø In liquids, isotropic tumbling averages the various orientations to zero Ø Simulated in a solid by spinning the sample Ø w µ ½(3 cos 2 q-1) Magic Angle (54. 7 o) averages to zero

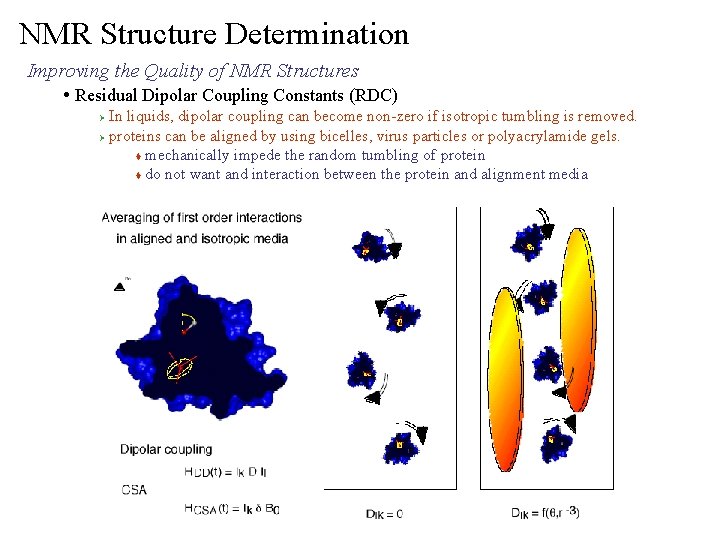
NMR Structure Determination Improving the Quality of NMR Structures • Residual Dipolar Coupling Constants (RDC) In liquids, dipolar coupling can become non-zero if isotropic tumbling is removed. Ø proteins can be aligned by using bicelles, virus particles or polyacrylamide gels. t mechanically impede the random tumbling of protein t do not want and interaction between the protein and alignment media Ø

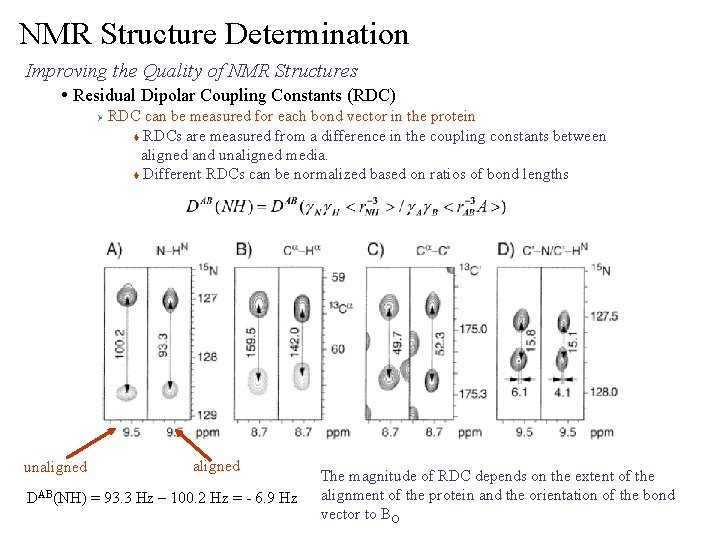
NMR Structure Determination Improving the Quality of NMR Structures • Residual Dipolar Coupling Constants (RDC) Ø unaligned RDC can be measured for each bond vector in the protein t RDCs are measured from a difference in the coupling constants between aligned and unaligned media. t Different RDCs can be normalized based on ratios of bond lengths aligned DAB(NH) = 93. 3 Hz – 100. 2 Hz = - 6. 9 Hz The magnitude of RDC depends on the extent of the alignment of the protein and the orientation of the bond vector to BO

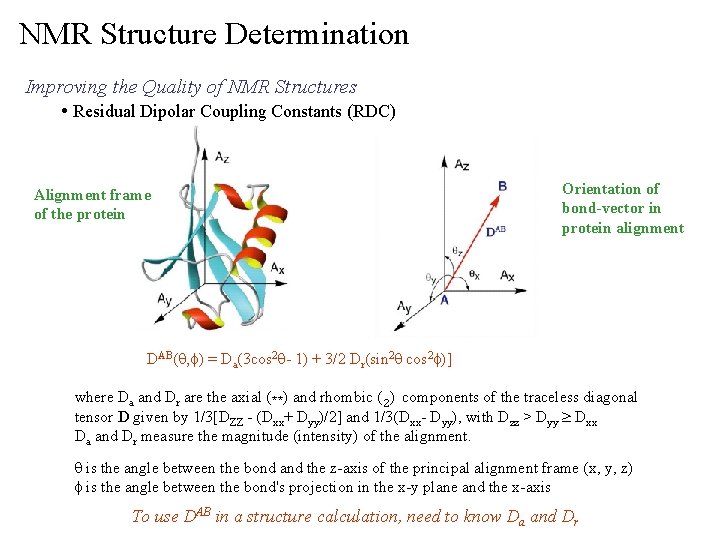
NMR Structure Determination Improving the Quality of NMR Structures • Residual Dipolar Coupling Constants (RDC) Alignment frame of the protein Orientation of bond-vector in protein alignment DAB(q, f) = Da(3 cos 2 q- 1) + 3/2 Dr(sin 2 q cos 2 f)] where Da and Dr are the axial (**) and rhombic (2) components of the traceless diagonal tensor D given by 1/3[DZZ - (Dxx+ Dyy)/2] and 1/3(Dxx- Dyy), with Dzz > Dyy ³ Dxx Da and Dr measure the magnitude (intensity) of the alignment. q is the angle between the bond and the z-axis of the principal alignment frame (x, y, z) f is the angle between the bond's projection in the x-y plane and the x-axis To use DAB in a structure calculation, need to know Da and Dr

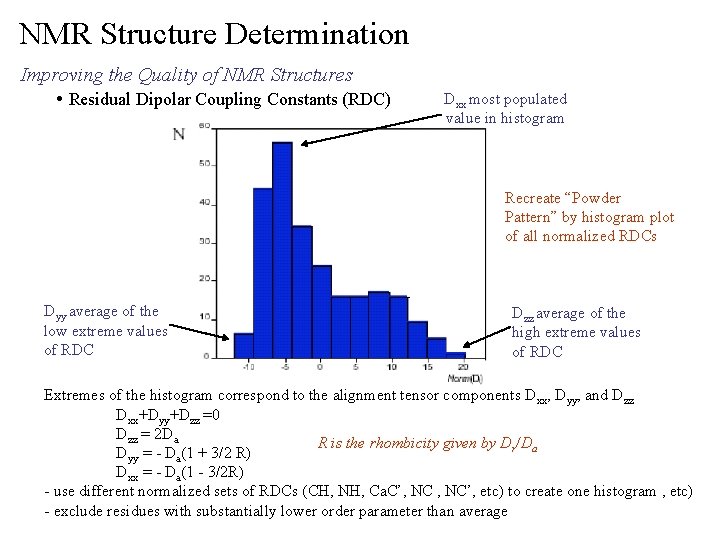
NMR Structure Determination Improving the Quality of NMR Structures • Residual Dipolar Coupling Constants (RDC) Dxx most populated value in histogram Recreate “Powder Pattern” by histogram plot of all normalized RDCs Dyy average of the low extreme values of RDC Dzz average of the high extreme values of RDC Extremes of the histogram correspond to the alignment tensor components Dxx, Dyy, and Dzz Dxx+Dyy+Dzz =0 Dzz = 2 Da R is the rhombicity given by Dr/Da Dyy = - Da(1 + 3/2 R) Dxx = - Da(1 - 3/2 R) - use different normalized sets of RDCs (CH, NH, Ca. C’, NC’, etc) to create one histogram , etc) - exclude residues with substantially lower order parameter than average

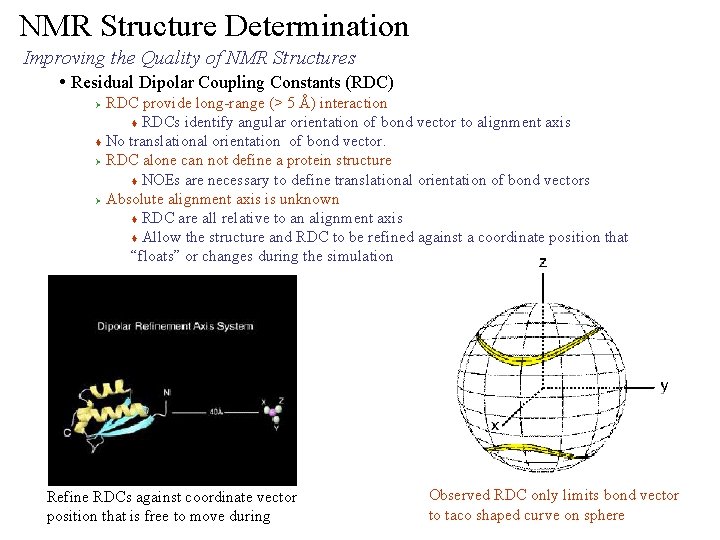
NMR Structure Determination Improving the Quality of NMR Structures • Residual Dipolar Coupling Constants (RDC) RDC provide long-range (> 5 Å) interaction t RDCs identify angular orientation of bond vector to alignment axis t No translational orientation of bond vector. Ø RDC alone can not define a protein structure t NOEs are necessary to define translational orientation of bond vectors Ø Absolute alignment axis is unknown t RDC are all relative to an alignment axis t Allow the structure and RDC to be refined against a coordinate position that “floats” or changes during the simulation Ø Refine RDCs against coordinate vector position that is free to move during Observed RDC only limits bond vector to taco shaped curve on sphere

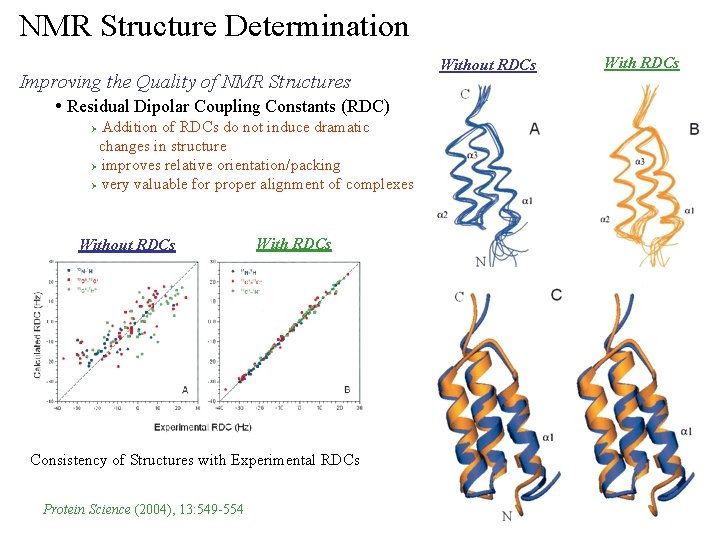
NMR Structure Determination Improving the Quality of NMR Structures • Residual Dipolar Coupling Constants (RDC) Addition of RDCs do not induce dramatic changes in structure Ø improves relative orientation/packing Ø very valuable for proper alignment of complexes Ø Without RDCs With RDCs Consistency of Structures with Experimental RDCs Protein Science (2004), 13: 549 -554 Without RDCs With RDCs

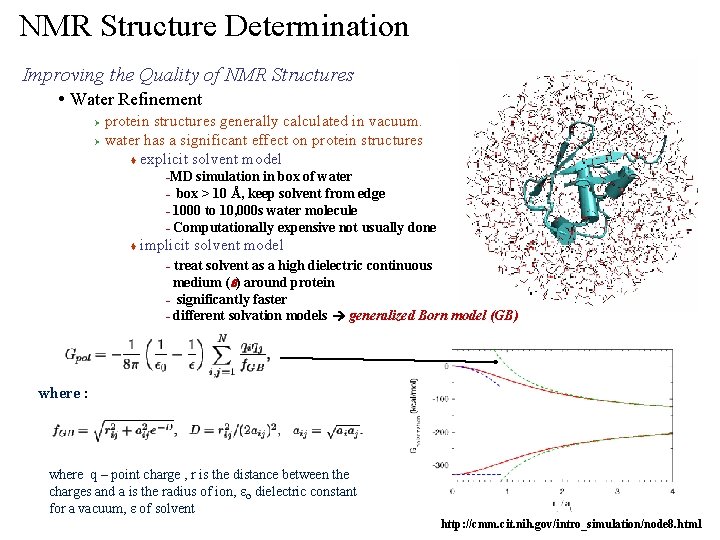
NMR Structure Determination Improving the Quality of NMR Structures • Water Refinement protein structures generally calculated in vacuum. Ø water has a significant effect on protein structures t explicit solvent model Ø MD simulation in box of water – box > 10 Å, keep solvent from edge – 1000 to 10, 000 s water molecule – Computationally expensive not usually done – t implicit solvent model treat solvent as a high dielectric continuous medium (e) around protein – significantly faster – different solvation models generalized Born model (GB) – where : where q – point charge , r is the distance between the charges and a is the radius of ion, eo dielectric constant for a vacuum, e of solvent http: //cmm. cit. nih. gov/intro_simulation/node 8. html

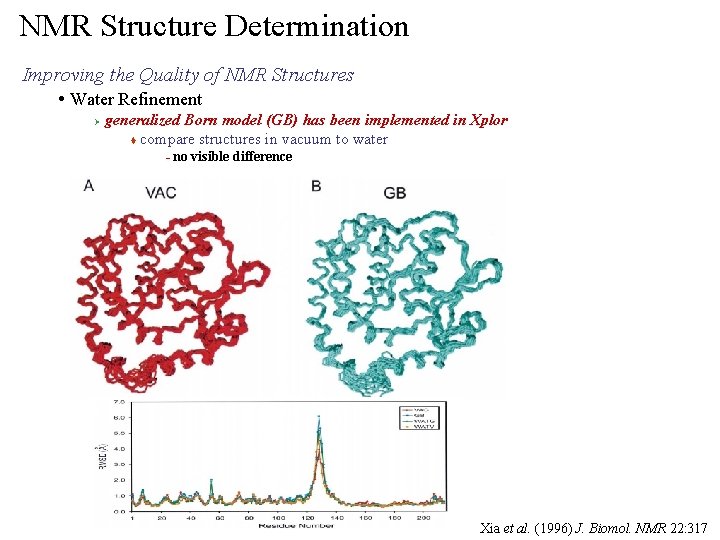
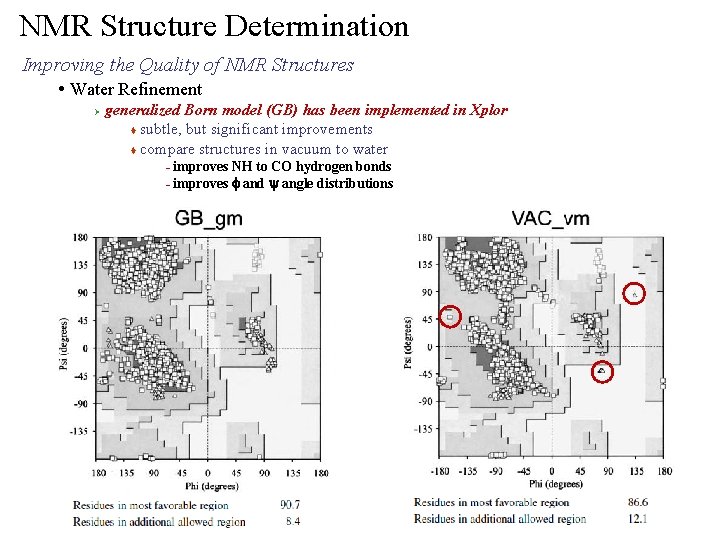
NMR Structure Determination Improving the Quality of NMR Structures • Water Refinement Ø generalized Born model (GB) has been implemented in Xplor t compare structures in vacuum to water – no visible difference Xia et al. (1996) J. Biomol. NMR 22: 317

NMR Structure Determination Improving the Quality of NMR Structures • Water Refinement Ø generalized Born model (GB) has been implemented in Xplor t subtle, but significant improvements t compare structures in vacuum to water improves NH to CO hydrogen bonds – improves f and y angle distributions –

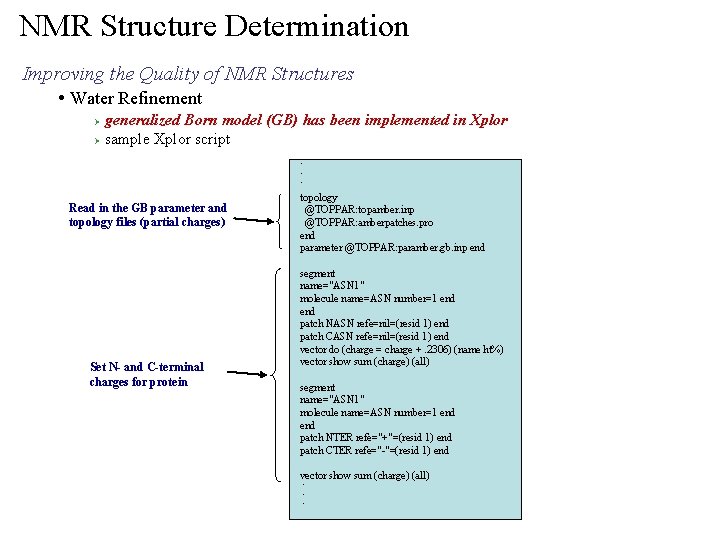
NMR Structure Determination Improving the Quality of NMR Structures • Water Refinement generalized Born model (GB) has been implemented in Xplor Ø sample Xplor script Ø . . . Read in the GB parameter and topology files (partial charges) Set N- and C-terminal charges for protein topology @TOPPAR: topamber. inp @TOPPAR: amberpatches. pro end parameter @TOPPAR: paramber. gb. inp end segment name="ASN 1" molecule name=ASN number=1 end patch NASN refe=nil=(resid 1) end patch CASN refe=nil=(resid 1) end vector do (charge = charge +. 2306) (name ht%) vector show sum (charge) (all) segment name="ASN 1" molecule name=ASN number=1 end patch NTER refe="+"=(resid 1) end patch CTER refe="-"=(resid 1) end vector show sum (charge) (all). . .

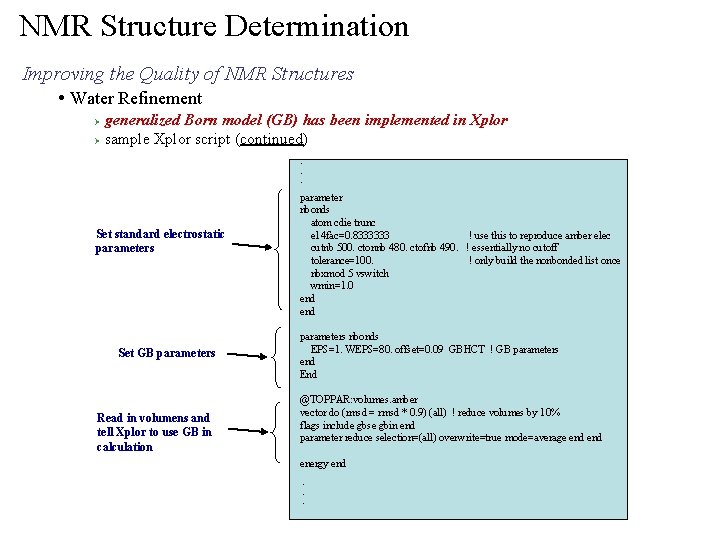
NMR Structure Determination Improving the Quality of NMR Structures • Water Refinement generalized Born model (GB) has been implemented in Xplor Ø sample Xplor script (continued) Ø . . . Set standard electrostatic parameters Set GB parameters Read in volumens and tell Xplor to use GB in calculation parameter nbonds atom cdie trunc e 14 fac=0. 8333333 ! use this to reproduce amber elec cutnb 500. ctonnb 480. ctofnb 490. ! essentially no cutoff tolerance=100. ! only build the nonbonded list once nbxmod 5 vswitch wmin=1. 0 end parameters nbonds EPS=1. WEPS=80. offset=0. 09 GBHCT ! GB parameters end End @TOPPAR: volumes. amber vector do (rmsd = rmsd * 0. 9) (all) ! reduce volumes by 10% flags include gbse gbin end parameter reduce selection=(all) overwrite=true mode=average end energy end. . .

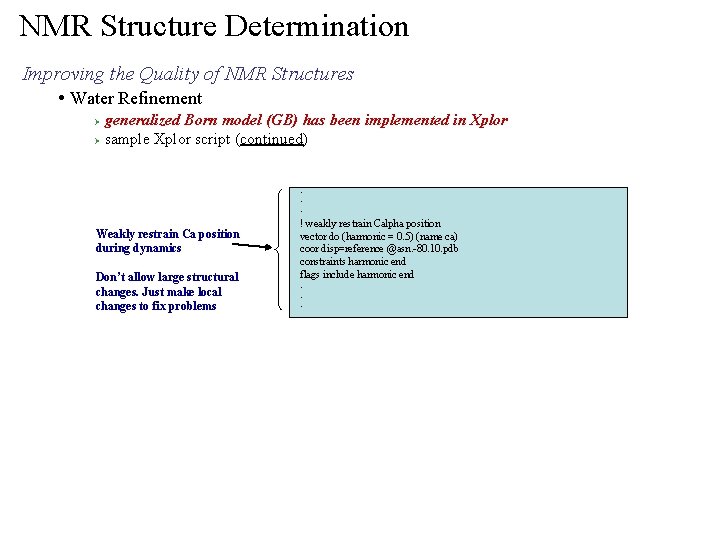
NMR Structure Determination Improving the Quality of NMR Structures • Water Refinement generalized Born model (GB) has been implemented in Xplor Ø sample Xplor script (continued) Ø . . . Weakly restrain Ca position during dynamics Don’t allow large structural changes. Just make local changes to fix problems ! weakly restrain Calpha position vector do (harmonic = 0. 5) (name ca) coor disp=reference @asn. -80. 10. pdb constraints harmonic end flags include harmonic end. . .