NMR Spectroscopy Spectrometer Hardware http www cis rit






- Slides: 22

NMR Spectroscopy Spectrometer -Hardware http: //www. cis. rit. edu/htbooks/nmr/inside. htm

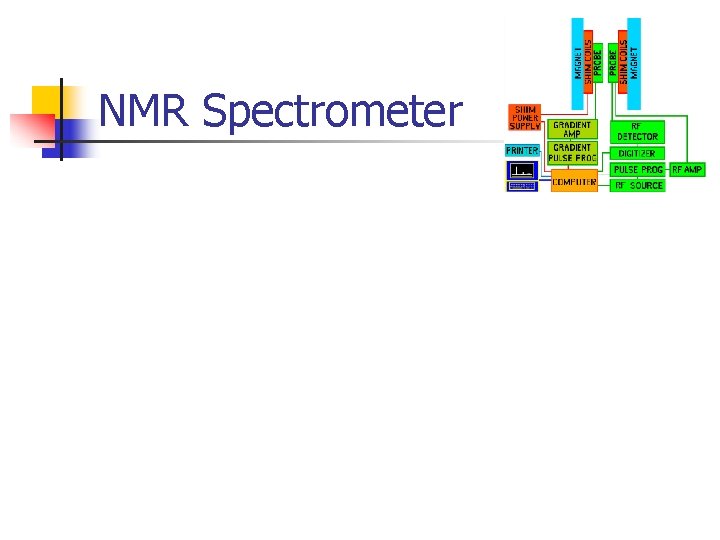
Spectrometer

NMR Spectrometer

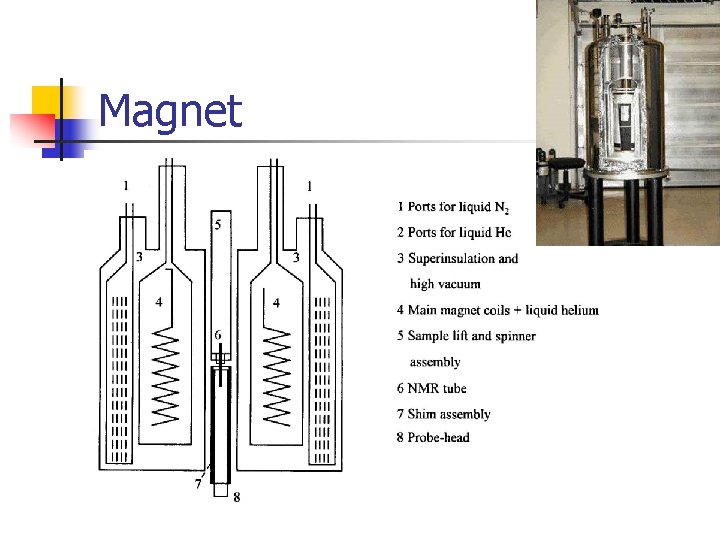
Magnet

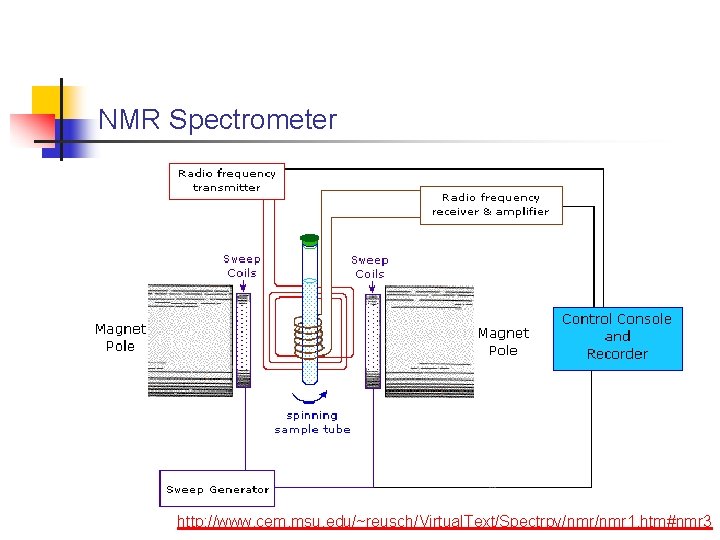
NMR Spectrometer http: //www. cem. msu. edu/~reusch/Virtual. Text/Spectrpy/nmr 1. htm#nmr 3

Observe Channel

Probe

Probe Requirement for probe small enough and symmetrically placed in magnet to keep field homogeneiety n provide means of locking n able to handle large RF voltages as well as receive and process weak FID sinals n

NMR Sample Preparation The majority of NMR samples are run in solution in NMR tubes http: //www. m-ltech. de/nmr-tubes. html

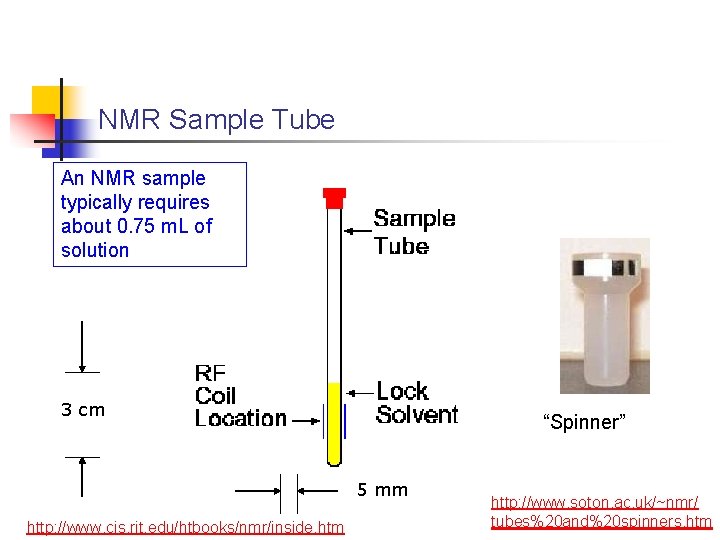
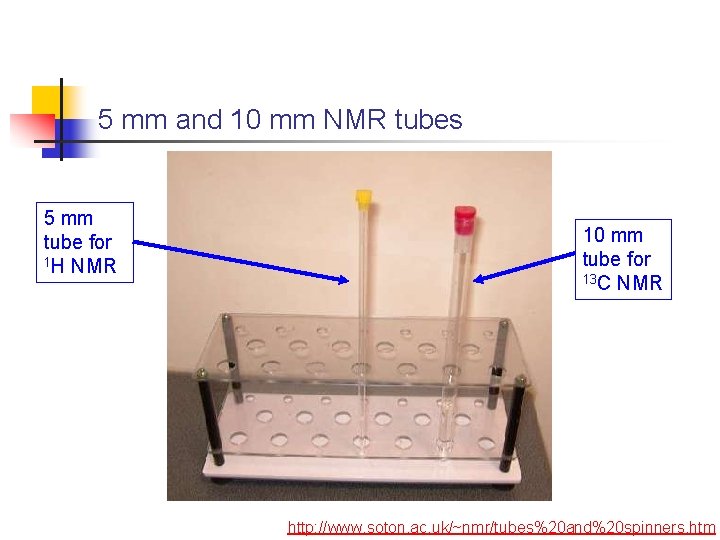
NMR Sample Tube An NMR sample typically requires about 0. 75 m. L of solution 3 cm “Spinner” 5 mm http: //www. cis. rit. edu/htbooks/nmr/inside. htm http: //www. soton. ac. uk/~nmr/ tubes%20 and%20 spinners. htm

5 mm and 10 mm NMR tubes 5 mm tube for 1 H NMR 10 mm tube for 13 C NMR http: //www. soton. ac. uk/~nmr/tubes%20 and%20 spinners. htm

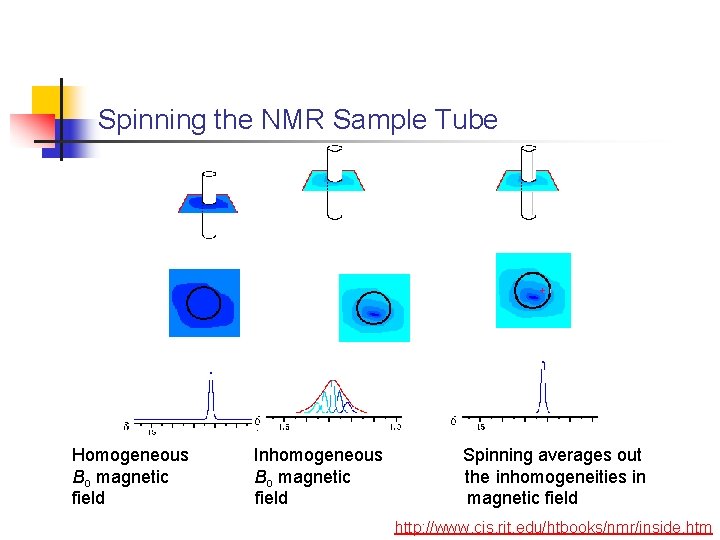
Spinning the NMR Sample Tube Homogeneous Bo magnetic field Inhomogeneous Bo magnetic field Spinning averages out the inhomogeneities in magnetic field http: //www. cis. rit. edu/htbooks/nmr/inside. htm

NMR Solvents • Most NMR spectra are recorded for compounds dissolved in a solvent. Therefore, signals will be observed for the solvent and this must be accounted for in solving spectral problems. • To avoid spectra dominated by the solvent signal, most 1 H NMR spectra are recorded in a deuterated solvent. However, deuteration is not "100%", so signals for the residual protons are observed. For chloroform as a solvent (CDCl 3), the residual signal is due to CHCl 3, so a singlet signal is observed at 7. 26 ppm. http: //www. chem. ucla. edu/~webspectra/Notes. On. Solvents. htm

NMR Solvents • It used to be common practice to add Me 4 Si (TMS), or related compounds, as an internal reference standard for 1 H and 13 C NMR spectra with the proton signal occurring at 0. 00 ppm and the carbon signal occurring at 0. 00 ppm in the 13 C NMR spectrum. However, modern spectrometers can "lock" on solvent signals, so addition of internal reference standards is not usually required.

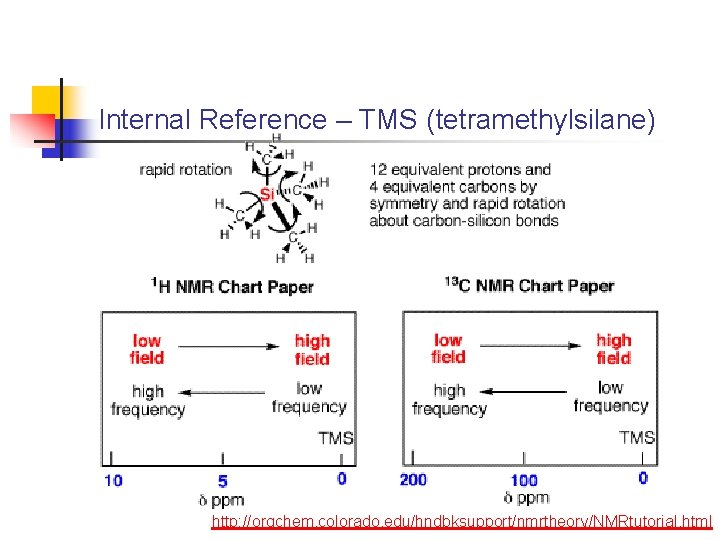
Internal Reference – TMS (tetramethylsilane) http: //orgchem. colorado. edu/hndbksupport/nmrtheory/NMRtutorial. html

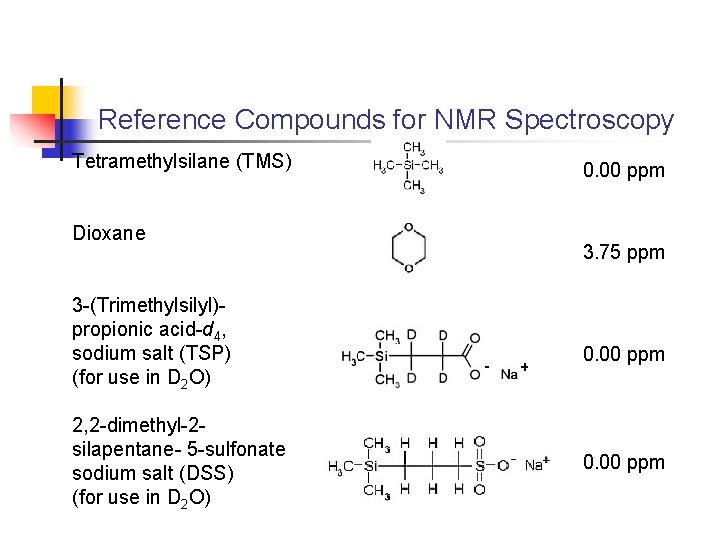
Reference Compounds for NMR Spectroscopy Tetramethylsilane (TMS) Dioxane 3 -(Trimethylsilyl)propionic acid-d 4, sodium salt (TSP) (for use in D 2 O) 2, 2 -dimethyl-2 silapentane- 5 -sulfonate sodium salt (DSS) (for use in D 2 O) 0. 00 ppm 3. 75 ppm 0. 00 ppm

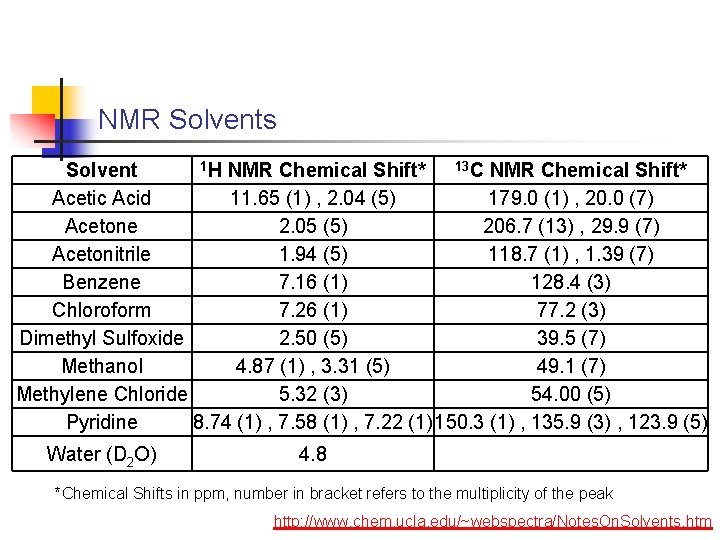
NMR Solvents 1 H NMR Chemical Shift* 13 C NMR Chemical Shift* Solvent Acetic Acid 11. 65 (1) , 2. 04 (5) 179. 0 (1) , 20. 0 (7) Acetone 2. 05 (5) 206. 7 (13) , 29. 9 (7) Acetonitrile 1. 94 (5) 118. 7 (1) , 1. 39 (7) Benzene 7. 16 (1) 128. 4 (3) Chloroform 7. 26 (1) 77. 2 (3) Dimethyl Sulfoxide 2. 50 (5) 39. 5 (7) Methanol 4. 87 (1) , 3. 31 (5) 49. 1 (7) Methylene Chloride 5. 32 (3) 54. 00 (5) Pyridine 8. 74 (1) , 7. 58 (1) , 7. 22 (1) 150. 3 (1) , 135. 9 (3) , 123. 9 (5) Water (D 2 O) 4. 8 *Chemical Shifts in ppm, number in bracket refers to the multiplicity of the peak http: //www. chem. ucla. edu/~webspectra/Notes. On. Solvents. htm

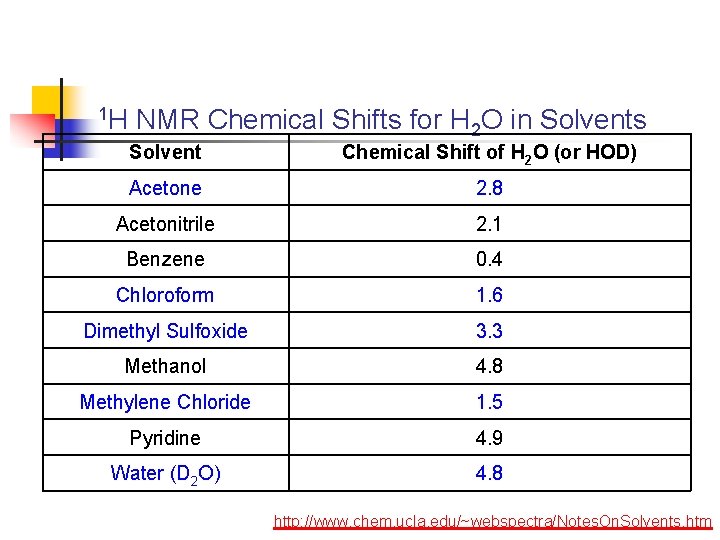
1 H NMR Chemical Shifts for H 2 O in Solvents Solvent Chemical Shift of H 2 O (or HOD) Acetone 2. 8 Acetonitrile 2. 1 Benzene 0. 4 Chloroform 1. 6 Dimethyl Sulfoxide 3. 3 Methanol 4. 8 Methylene Chloride 1. 5 Pyridine 4. 9 Water (D 2 O) 4. 8 http: //www. chem. ucla. edu/~webspectra/Notes. On. Solvents. htm

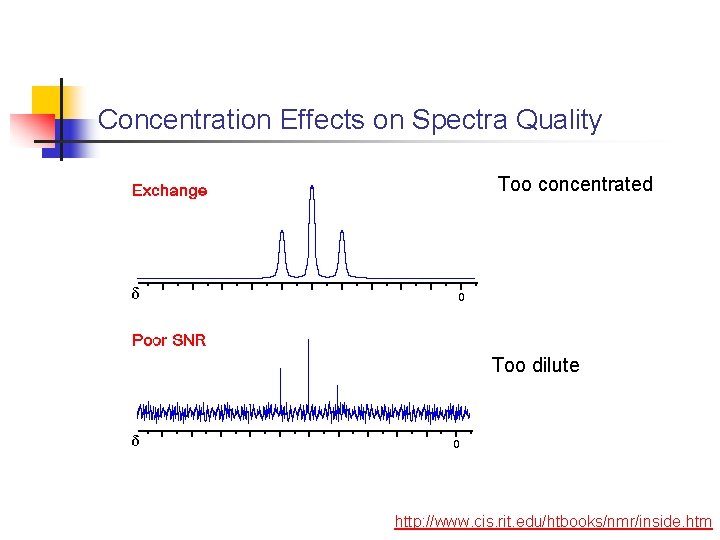
Concentration Effects on Spectra Quality Too concentrated Too dilute http: //www. cis. rit. edu/htbooks/nmr/inside. htm

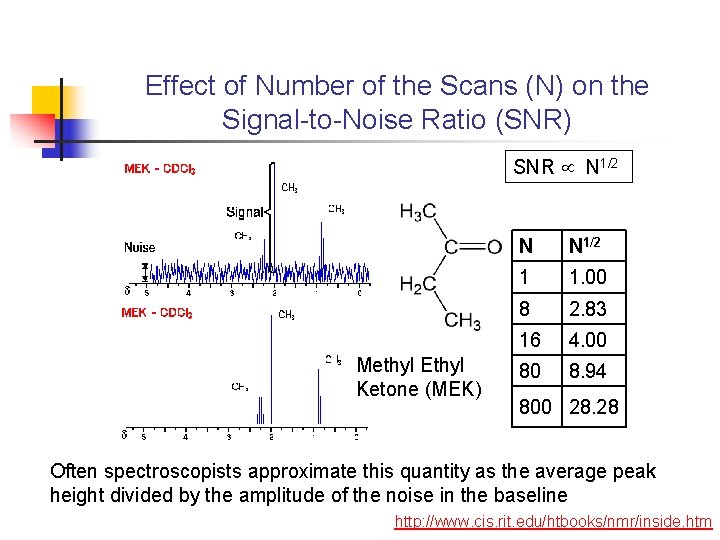
Effect of Number of the Scans (N) on the Signal-to-Noise Ratio (SNR) SNR N 1/2 Methyl Ethyl Ketone (MEK) N N 1/2 1 1. 00 8 2. 83 16 4. 00 80 8. 94 800 28. 28 Often spectroscopists approximate this quantity as the average peak height divided by the amplitude of the noise in the baseline http: //www. cis. rit. edu/htbooks/nmr/inside. htm

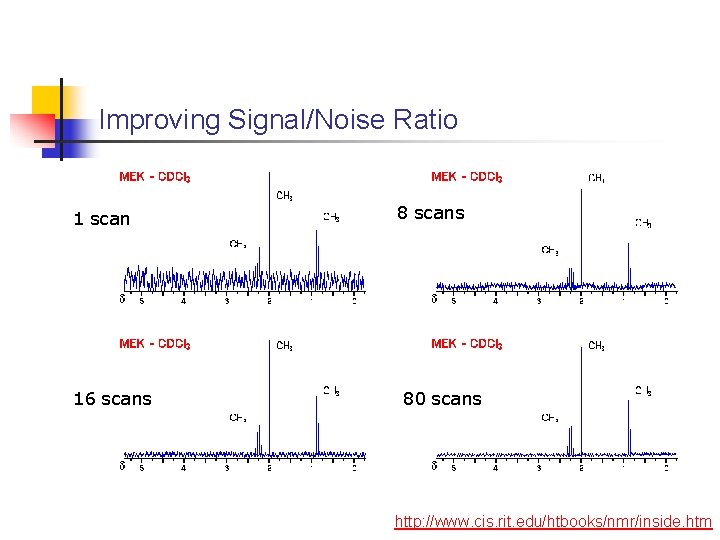
Improving Signal/Noise Ratio 1 scan 16 scans 80 scans http: //www. cis. rit. edu/htbooks/nmr/inside. htm

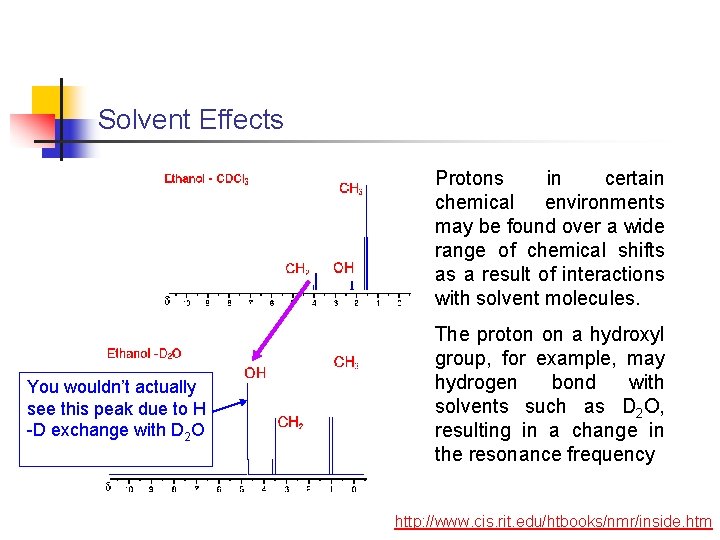
Solvent Effects Protons in certain chemical environments may be found over a wide range of chemical shifts as a result of interactions with solvent molecules. You wouldn’t actually see this peak due to H -D exchange with D 2 O The proton on a hydroxyl group, for example, may hydrogen bond with solvents such as D 2 O, resulting in a change in the resonance frequency http: //www. cis. rit. edu/htbooks/nmr/inside. htm