NMR Spectroscopy Interpretation of NMR spectrum II Nuclear
- Slides: 20

NMR Spectroscopy Interpretation of NMR spectrum II

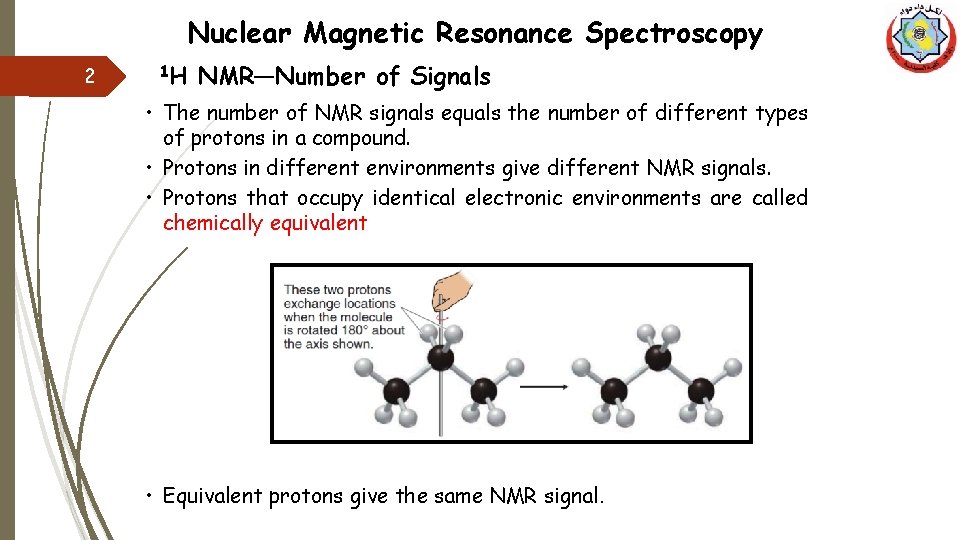
Nuclear Magnetic Resonance Spectroscopy 2 1 H NMR—Number of Signals • The number of NMR signals equals the number of different types of protons in a compound. • Protons in different environments give different NMR signals. • Protons that occupy identical electronic environments are called chemically equivalent • Equivalent protons give the same NMR signal.

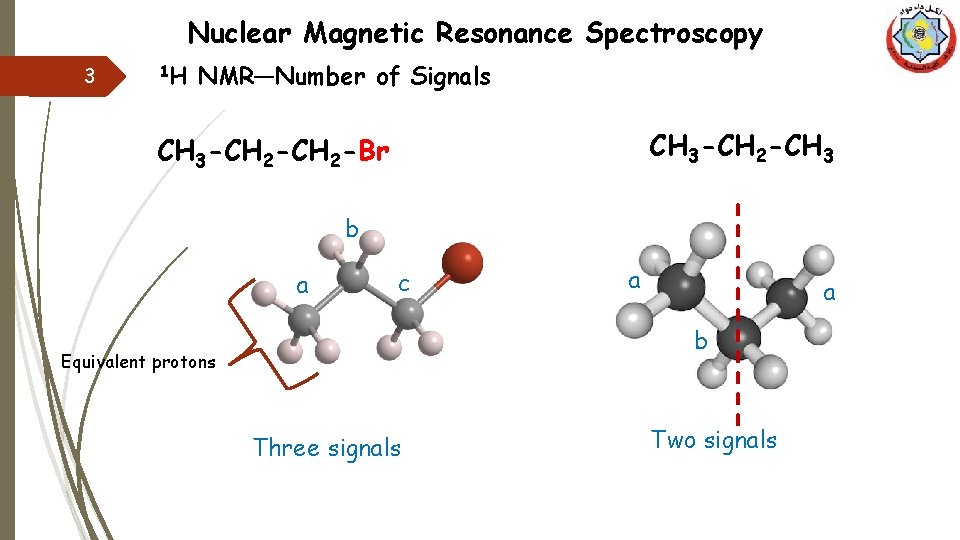
Nuclear Magnetic Resonance Spectroscopy 3 1 H NMR—Number of Signals CH 3 -CH 2 -CH 3 -CH 2 -Br b a c a a b Equivalent protons Three signals Two signals

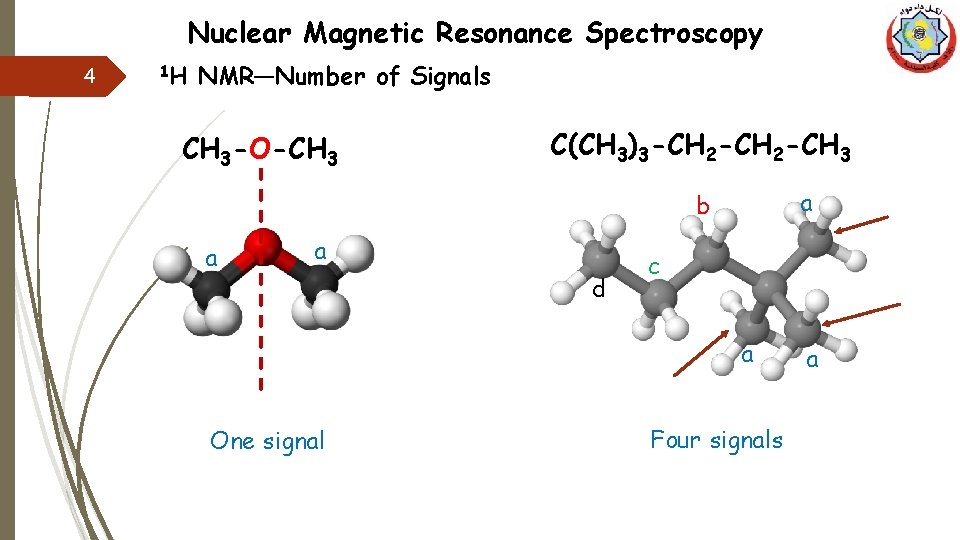
Nuclear Magnetic Resonance Spectroscopy 4 1 H NMR—Number of Signals CH 3 -O-CH 3 C(CH 3)3 -CH 2 -CH 3 a b a a d c a One signal Four signals a

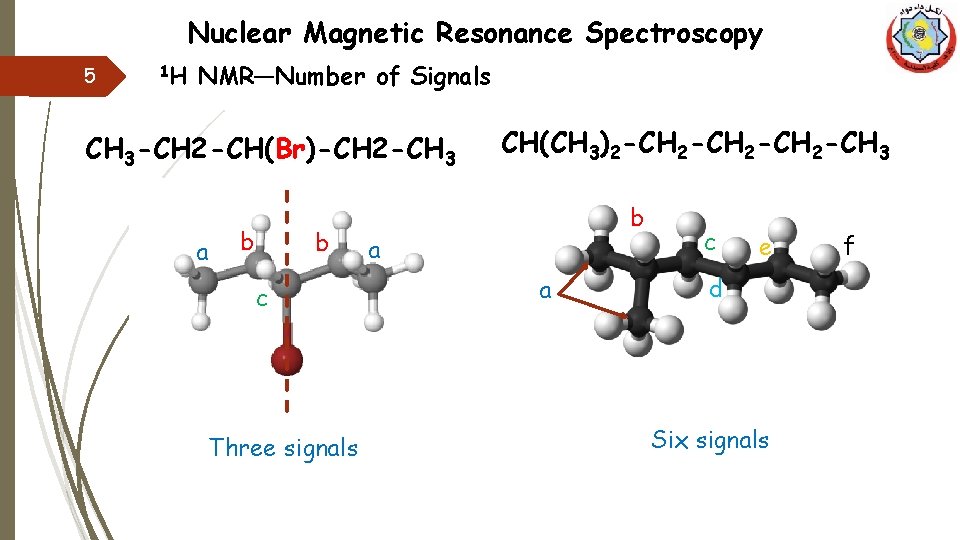
Nuclear Magnetic Resonance Spectroscopy 5 1 H NMR—Number of Signals CH 3 -CH 2 -CH(Br)-CH 2 -CH 3 aa b ab c Three signals CH(CH 3)2 -CH 3 b a a c e d Six signals f

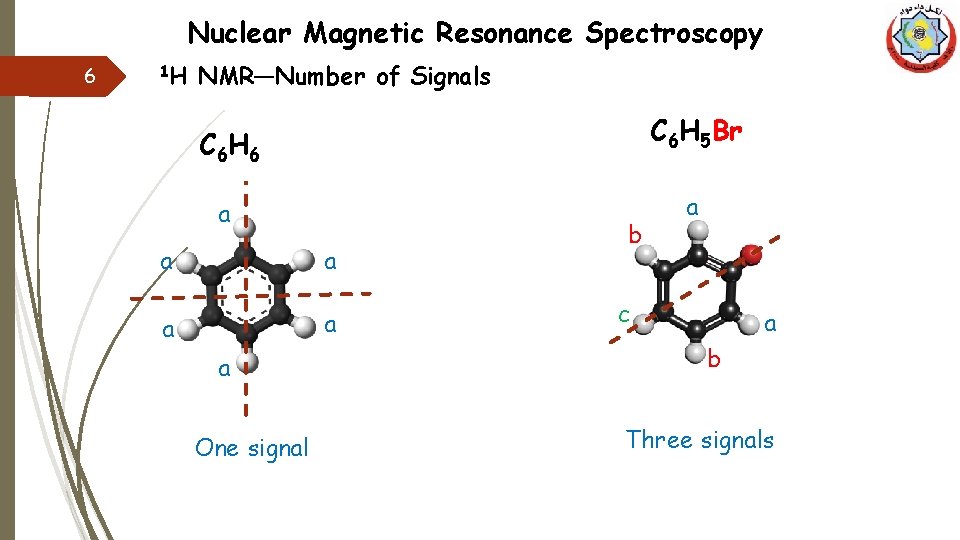
Nuclear Magnetic Resonance Spectroscopy 6 1 H NMR—Number of Signals C 6 H 5 Br C 6 H 6 a a a One signal b a c a b Three signals

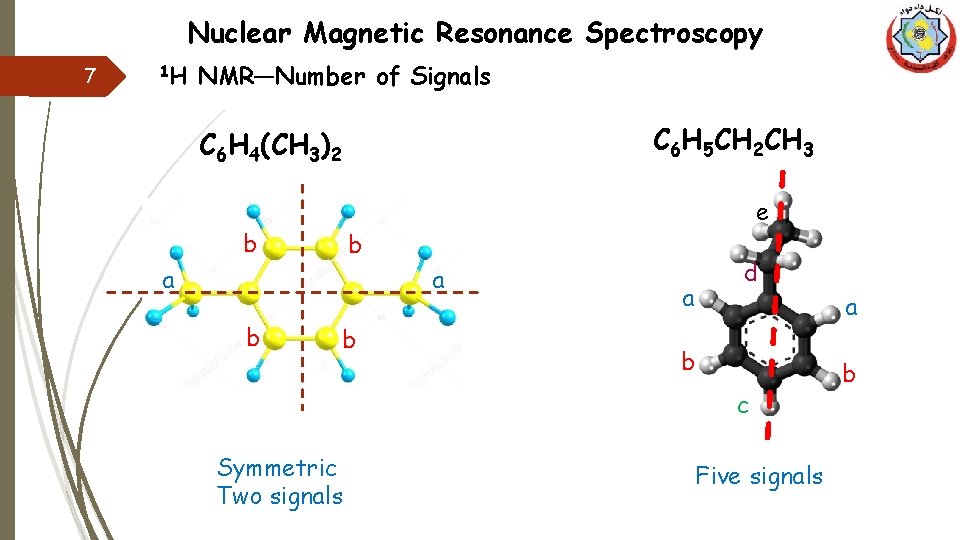
Nuclear Magnetic Resonance Spectroscopy 7 1 H NMR—Number of Signals C 6 H 5 CH 2 CH 3 C 6 H 4(CH 3)2 b e b a a b b a d a b c Symmetric Two signals Five signals b

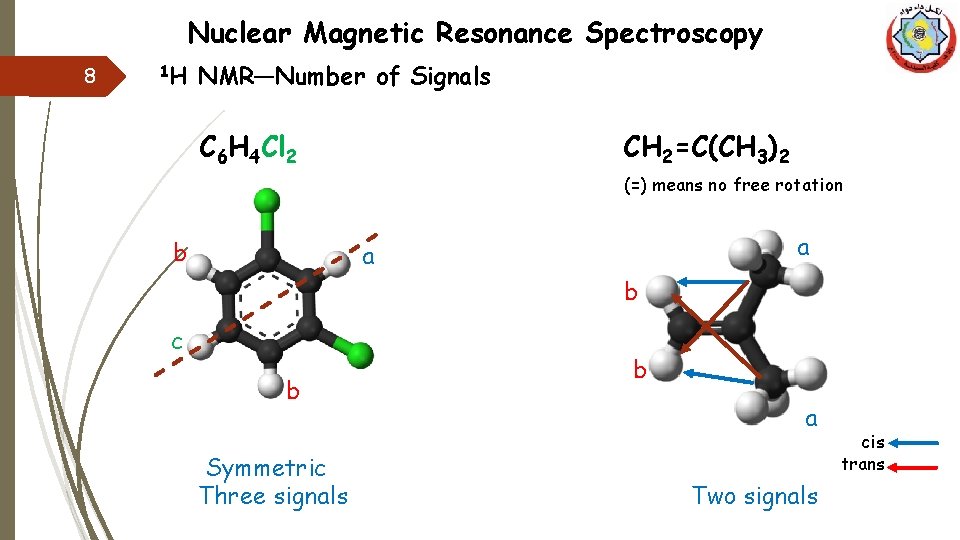
Nuclear Magnetic Resonance Spectroscopy 8 1 H NMR—Number of Signals C 6 H 4 Cl 2 CH 2=C(CH 3)2 (=) means no free rotation b a a b c b Symmetric Three signals b a Two signals cis trans

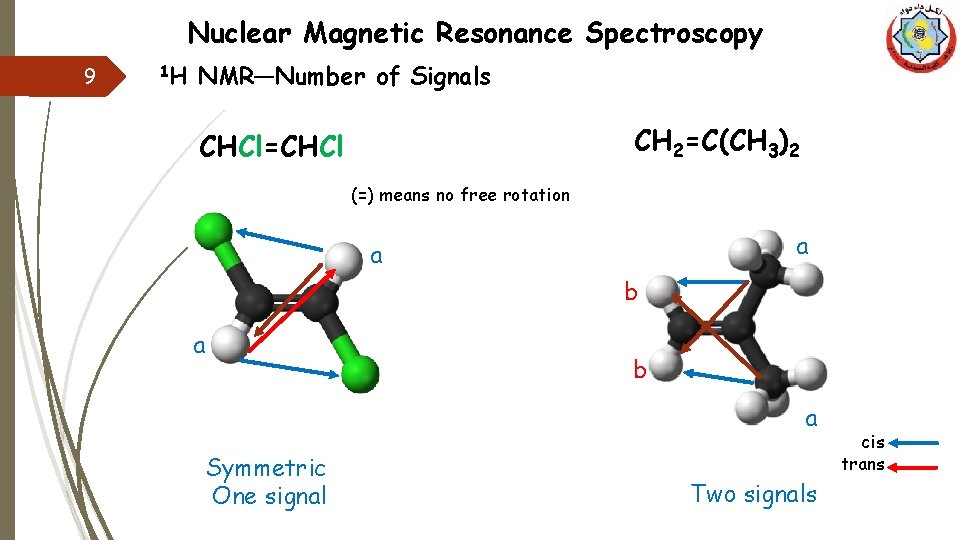
Nuclear Magnetic Resonance Spectroscopy 9 1 H NMR—Number of Signals CH 2=C(CH 3)2 CHCl=CHCl (=) means no free rotation a a b a Symmetric One signal Two signals cis trans

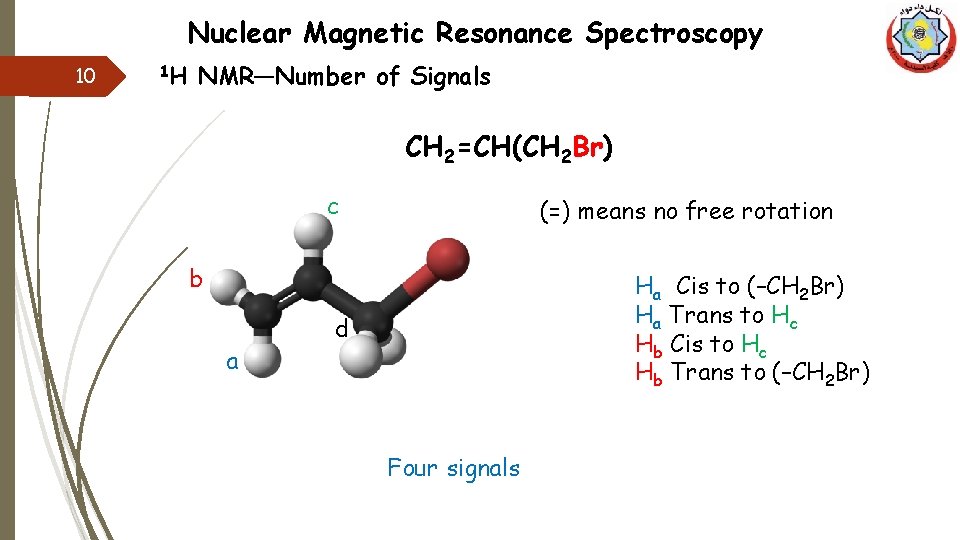
Nuclear Magnetic Resonance Spectroscopy 10 1 H NMR—Number of Signals CH 2=CH(CH 2 Br) c (=) means no free rotation b a Ha Cis to (–CH 2 Br) Ha Trans to Hc Hb Cis to Hc Hb Trans to (–CH 2 Br) d Four signals

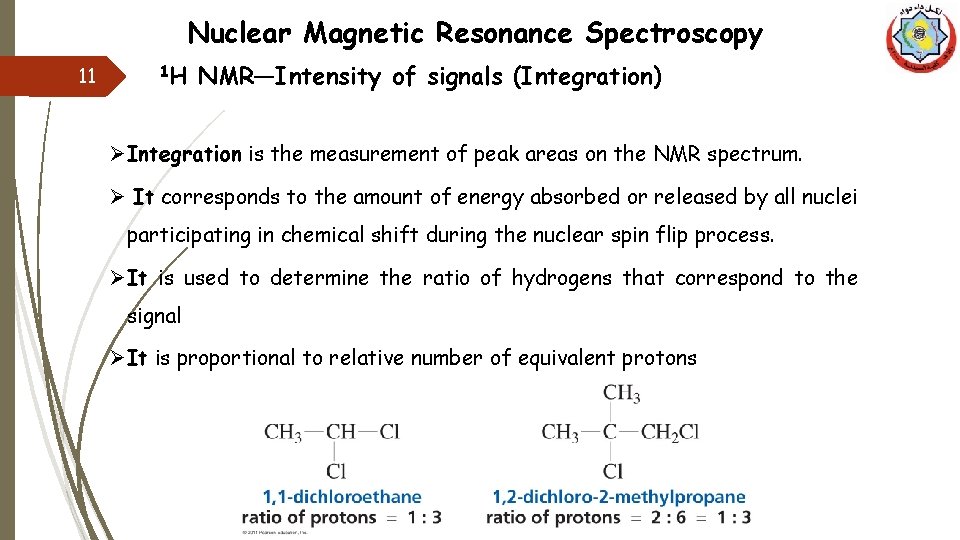
Nuclear Magnetic Resonance Spectroscopy 11 1 H NMR—Intensity of signals (Integration) ØIntegration is the measurement of peak areas on the NMR spectrum. Ø It corresponds to the amount of energy absorbed or released by all nuclei participating in chemical shift during the nuclear spin flip process. ØIt is used to determine the ratio of hydrogens that correspond to the signal ØIt is proportional to relative number of equivalent protons

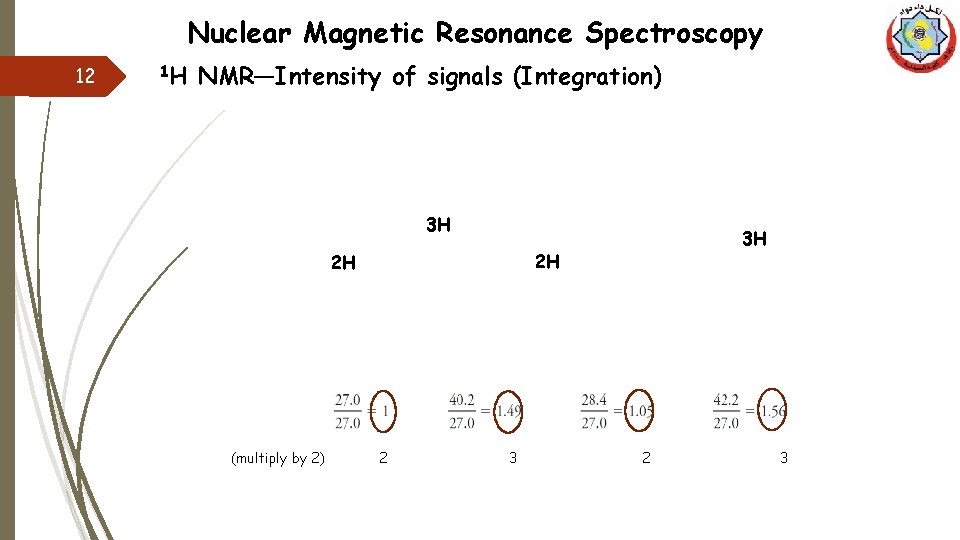
Nuclear Magnetic Resonance Spectroscopy 12 1 H NMR—Intensity of signals (Integration) 3 H 2 H 2 H (multiply by 2) 3 H 2 3

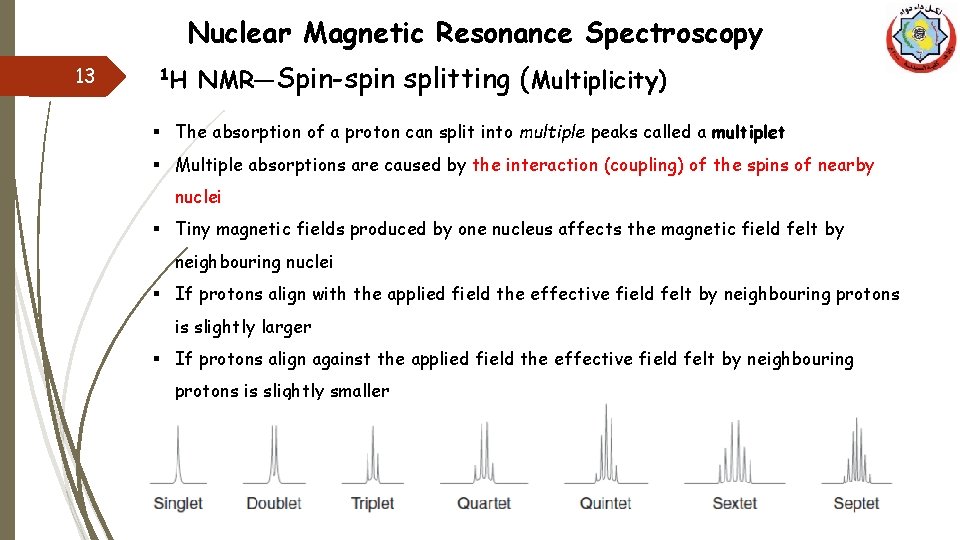
Nuclear Magnetic Resonance Spectroscopy 13 1 H NMR—Spin-spin splitting (Multiplicity) § The absorption of a proton can split into multiple peaks called a multiplet § Multiple absorptions are caused by the interaction (coupling) of the spins of nearby nuclei § Tiny magnetic fields produced by one nucleus affects the magnetic field felt by neighbouring nuclei § If protons align with the applied field the effective field felt by neighbouring protons is slightly larger § If protons align against the applied field the effective field felt by neighbouring protons is slightly smaller

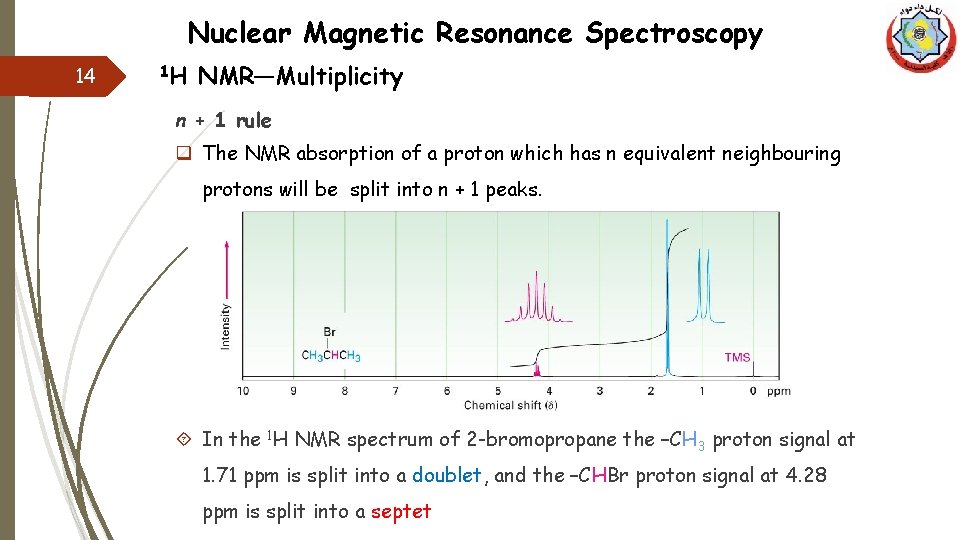
Nuclear Magnetic Resonance Spectroscopy 14 1 H NMR—Multiplicity n + 1 rule q The NMR absorption of a proton which has n equivalent neighbouring protons will be split into n + 1 peaks. In the 1 H NMR spectrum of 2 -bromopropane the –CH 3 proton signal at 1. 71 ppm is split into a doublet, and the –CHBr proton signal at 4. 28 ppm is split into a septet

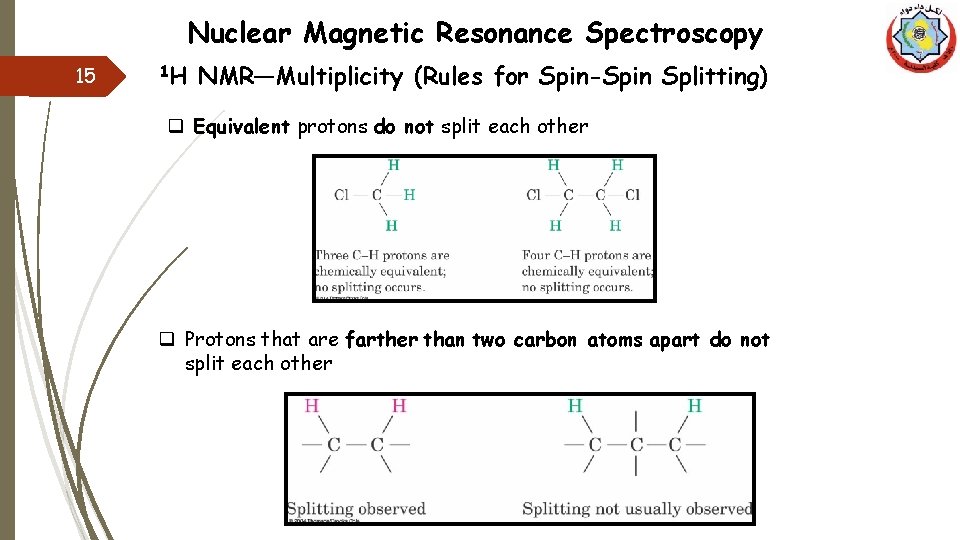
Nuclear Magnetic Resonance Spectroscopy 15 1 H NMR—Multiplicity (Rules for Spin-Spin Splitting) q Equivalent protons do not split each other q Protons that are farther than two carbon atoms apart do not split each other

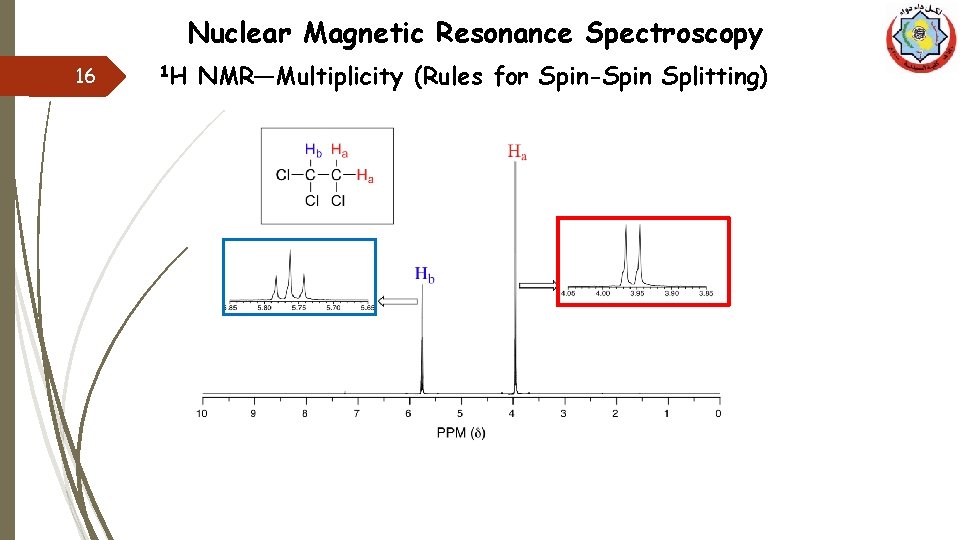
Nuclear Magnetic Resonance Spectroscopy 16 1 H NMR—Multiplicity (Rules for Spin-Spin Splitting)

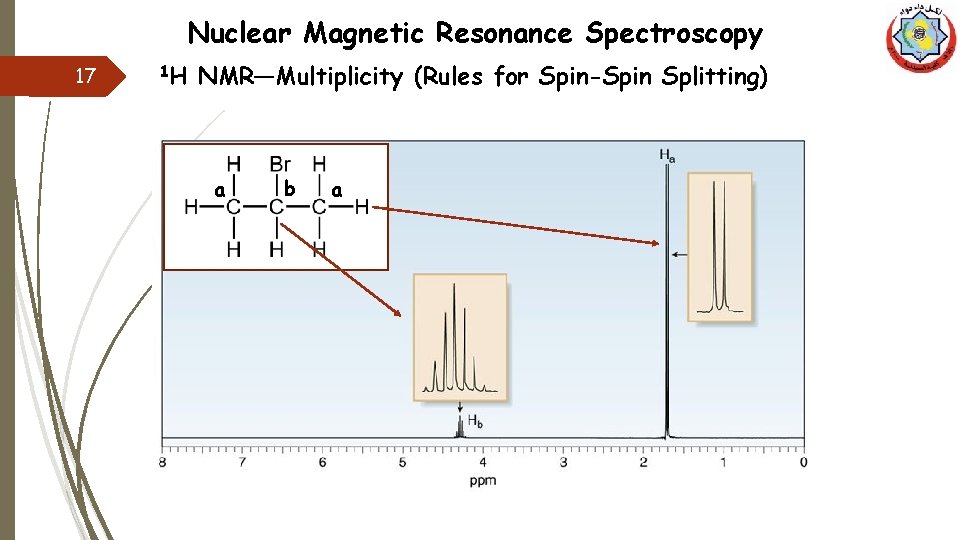
Nuclear Magnetic Resonance Spectroscopy 17 1 H NMR—Multiplicity (Rules for Spin-Spin Splitting) a b a

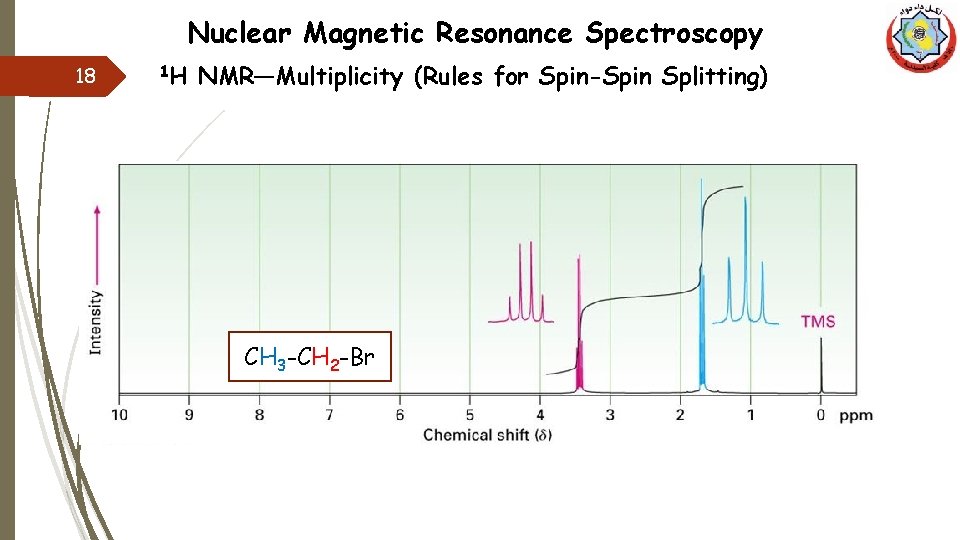
Nuclear Magnetic Resonance Spectroscopy 18 1 H NMR—Multiplicity (Rules for Spin-Spin Splitting) CH 3 -CH 2 -Br

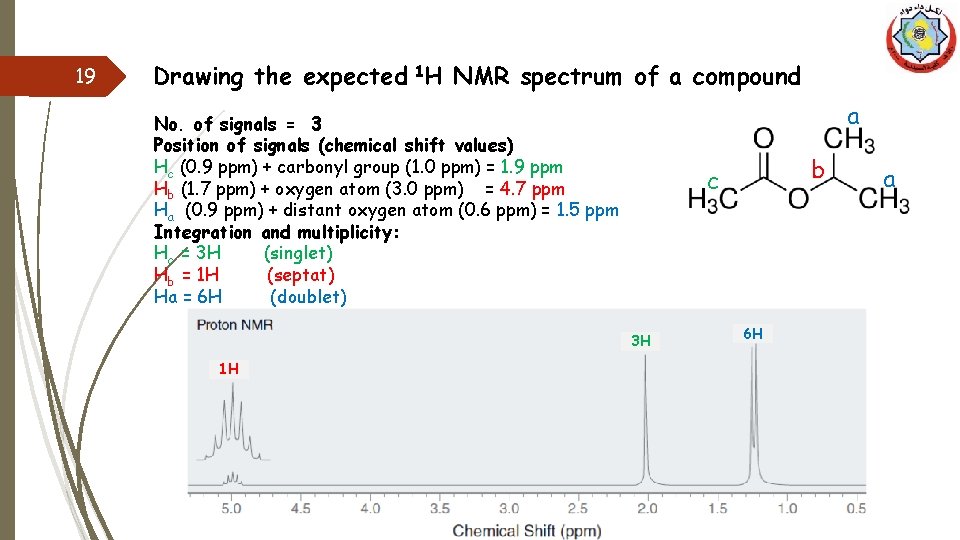
19 Drawing the expected 1 H NMR spectrum of a compound a No. of signals = 3 Position of signals (chemical shift values) Hc (0. 9 ppm) + carbonyl group (1. 0 ppm) = 1. 9 ppm Hb (1. 7 ppm) + oxygen atom (3. 0 ppm) = 4. 7 ppm Ha (0. 9 ppm) + distant oxygen atom (0. 6 ppm) = 1. 5 ppm Integration and multiplicity: Hc = 3 H (singlet) Hb = 1 H (septat) Ha = 6 H (doublet) 3 H 1 H b c 6 H a

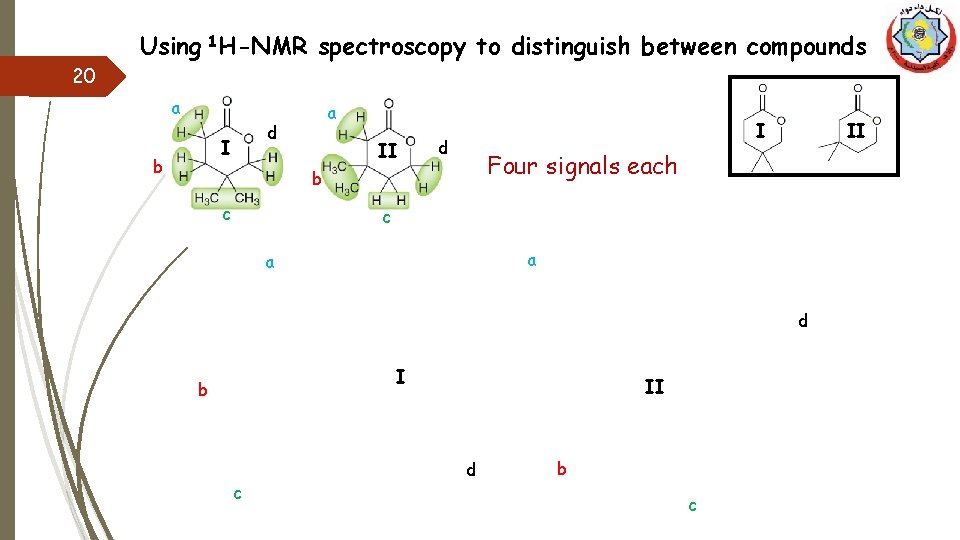
Using 1 H-NMR spectroscopy to distinguish between compounds 20 a d I b a II I d Four signals each b c II c a a d I b c II d b c