NMR II Pulse sequence and NMR experiments Instructor




- Slides: 58

NMR II- Pulse sequence and NMR experiments Instructor: Tai-huang Huang (黃太煌) 中央研究院生物醫學科學研究所 Tel. (886)-2 -2652 -3036; E. mail: bmthh@ibms. sinica. edu. tw Web site: www. nmr. ibms. sinica. edu. tw/~thh/biophysics/NMR-2. ppt Reference: Cavanagh, J. et al. , “Protein NMR Spectroscopy-Principles and Practice”, Academic Press, 1996.

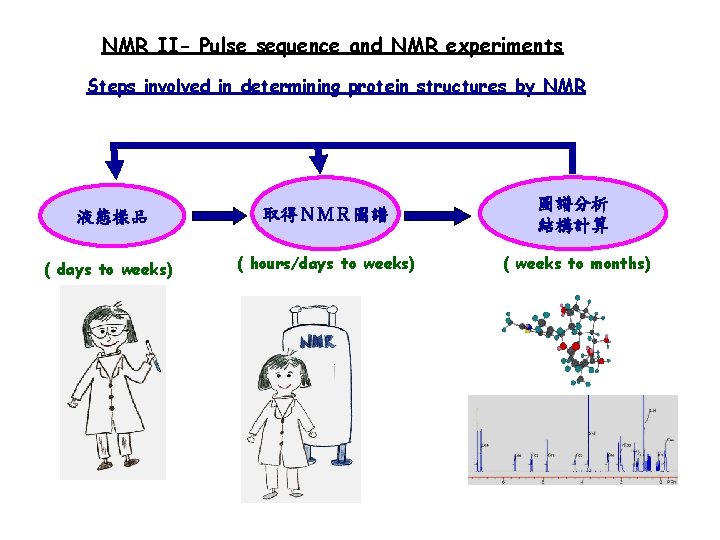
NMR II- Pulse sequence and NMR experiments Steps involved in determining protein structures by NMR 液態樣品 取得NMR圖譜 圖譜分析 結構計算 ( days to weeks) ( hours/days to weeks) ( weeks to months)

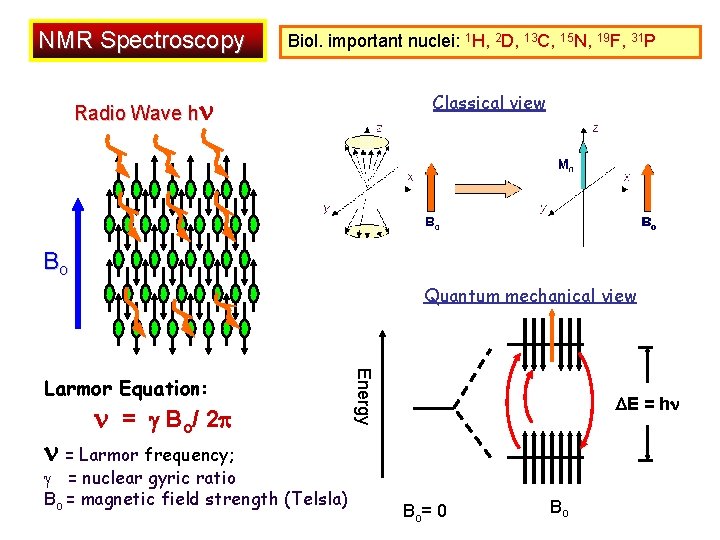
NMR Spectroscopy Biol. important nuclei: 1 H, 2 D, 13 C, 15 N, 19 F, 31 P Classical view Radio Wave h Bo Quantum mechanical view = Bo/ 2 Energy Larmor Equation: E = h = Larmor frequency; = nuclear gyric ratio Bo = magnetic field strength (Telsla) Bo= 0 Bo

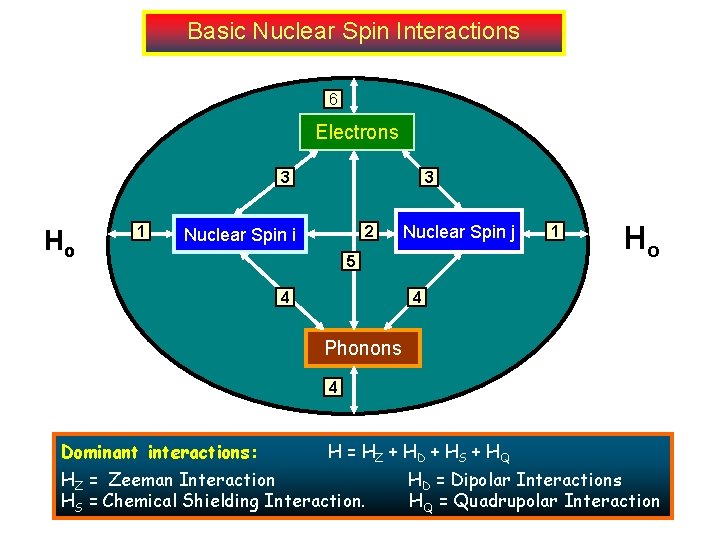
Basic Nuclear Spin Interactions 6 Electrons 3 Ho 1 3 2 Nuclear Spin i Nuclear Spin j 5 4 1 Ho 4 Phonons 4 Dominant interactions: . H = HZ + HD + HS + HQ HZ = Zeeman Interaction HS = Chemical Shielding Interaction. HD = Dipolar Interactions HQ = Quadrupolar Interaction

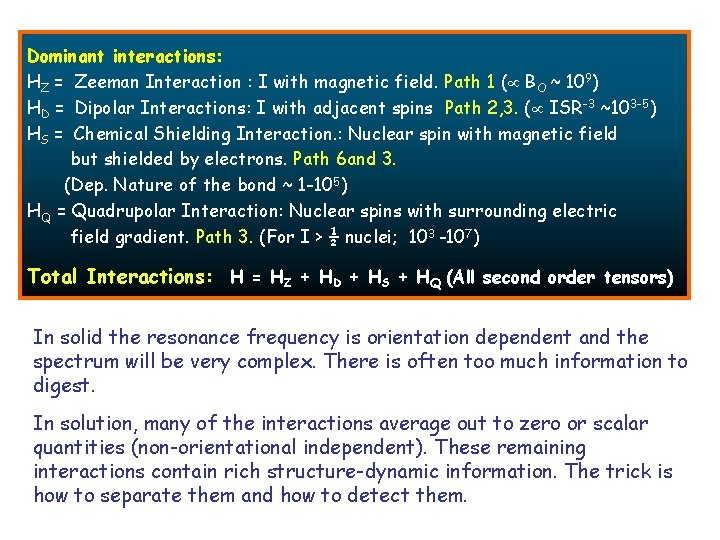
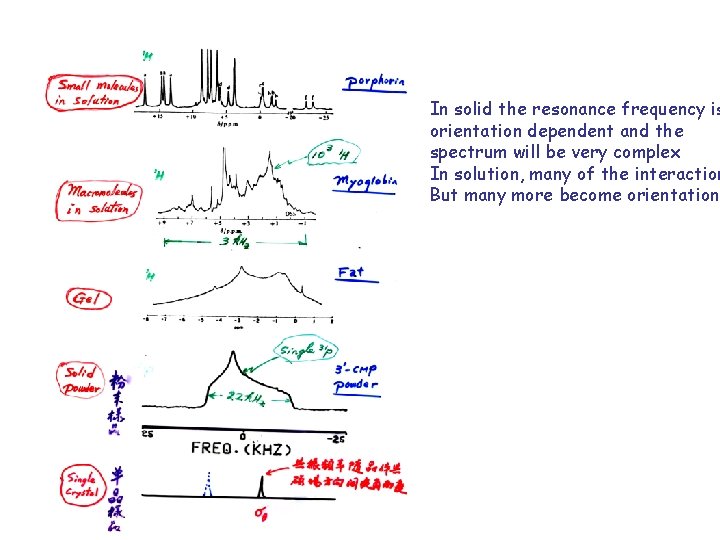
Dominant interactions: HZ = Zeeman Interaction : I with magnetic field. Path 1 ( BO ~ 109) HD = Dipolar Interactions: I with adjacent spins Path 2, 3. ( ISR-3 ~103 -5) HS = Chemical Shielding Interaction. : Nuclear spin with magnetic field but shielded by electrons. Path 6 and 3. (Dep. Nature of the bond ~ 1 -105) HQ = Quadrupolar Interaction: Nuclear spins with surrounding electric field gradient. Path 3. (For I > ½ nuclei; 103 -107). Total Interactions: H = HZ + HD + HS + HQ (All second order tensors) In solid the resonance frequency is orientation dependent and the spectrum will be very complex. There is often too much information to digest. In solution, many of the interactions average out to zero or scalar quantities (non-orientational independent). These remaining interactions contain rich structure-dynamic information. The trick is how to separate them and how to detect them.

In solid the resonance frequency is orientation dependent and the spectrum will be very complex In solution, many of the interaction But many more become orientationa

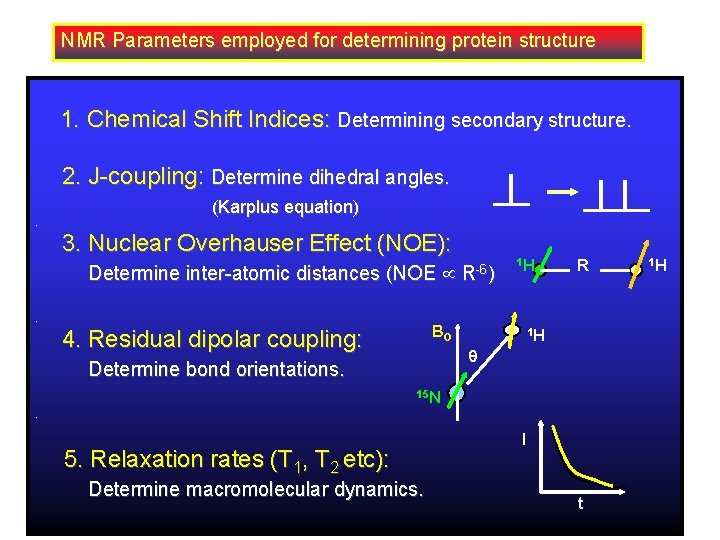
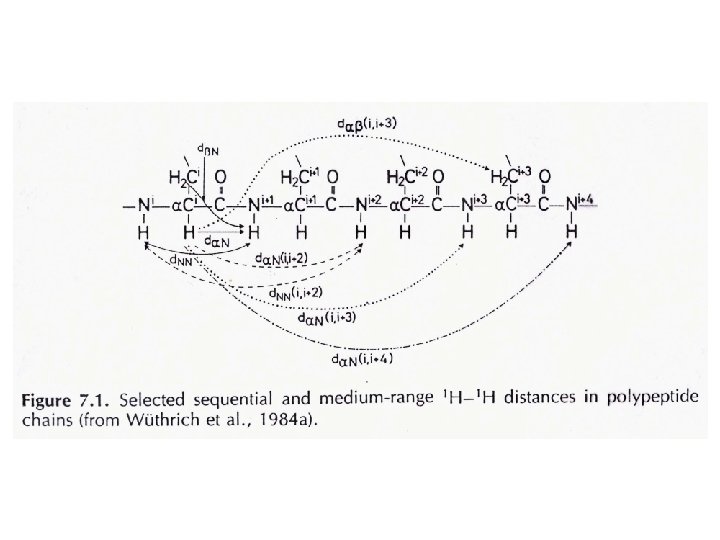
NMR Parameters employed for determining protein structure 1. Chemical Shift Indices: Determining secondary structure. 2. J-coupling: Determine dihedral angles. (Karplus equation). 3. Nuclear Overhauser Effect (NOE): Determine inter-atomic distances (NOE . R-6) 1 H BO 4. Residual dipolar coupling: R 1 H Determine bond orientations. 15 N. 5. Relaxation rates (T 1, T 2 etc): Determine macromolecular dynamics. I t 1 H

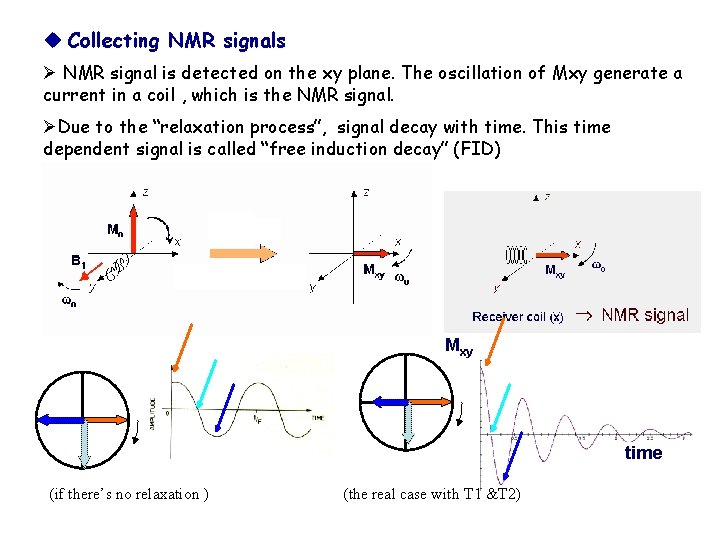
Collecting NMR signals Ø NMR signal is detected on the xy plane. The oscillation of Mxy generate a current in a coil , which is the NMR signal. ØDue to the “relaxation process”, signal decay with time. This time dependent signal is called “free induction decay” (FID) Mxy time (if there’s no relaxation ) (the real case with T 1 &T 2)

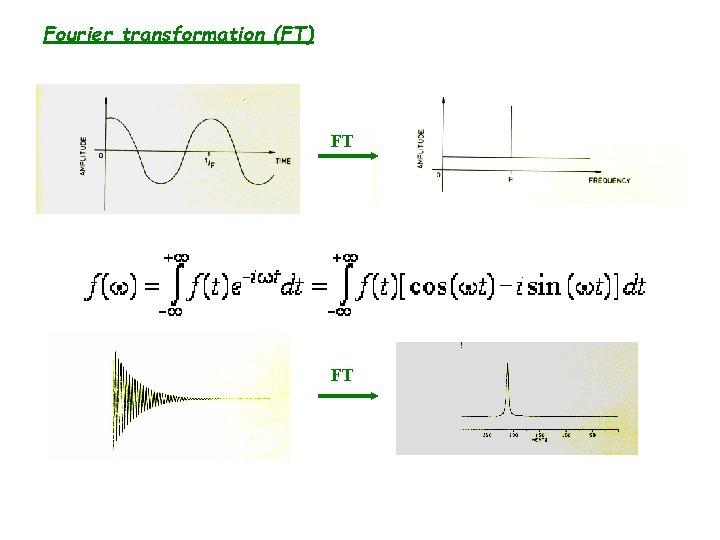
Fourier transformation (FT) FT FT

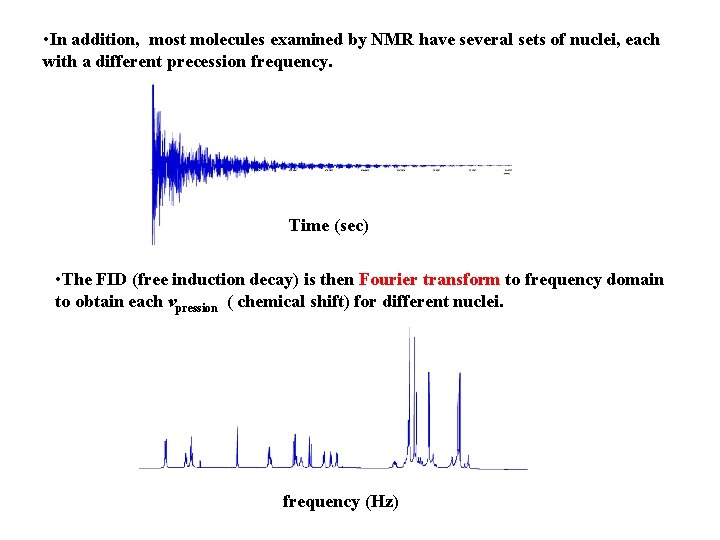
• In addition, most molecules examined by NMR have several sets of nuclei, each with a different precession frequency. Time (sec) • The FID (free induction decay) is then Fourier transform to frequency domain to obtain each vpression ( chemical shift) for different nuclei. frequency (Hz)


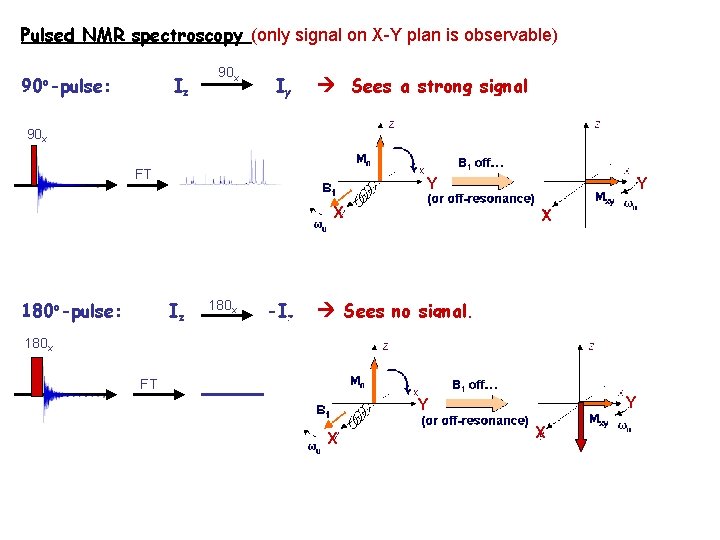
Pulsed NMR spectroscopy (only signal on X-Y plan is observable) 90 o-pulse: Iz 90 x Iy Sees a strong signal 90 x FT Y X 180 o-pulse: Iz 180 x -Iz Y X Sees no signal. 180 x FT Y Y X X

Pulsed NMR spectroscopy (only signal on X-Y plan is observable) -90 o-pulse: Iz 90 x Iy Sees a strong negative signal -90 x (same as 270 x) FT Y X -180 o-pulse: Iz 180 x -Iz Y X Sees no signal. -180 x FT Y Y X X

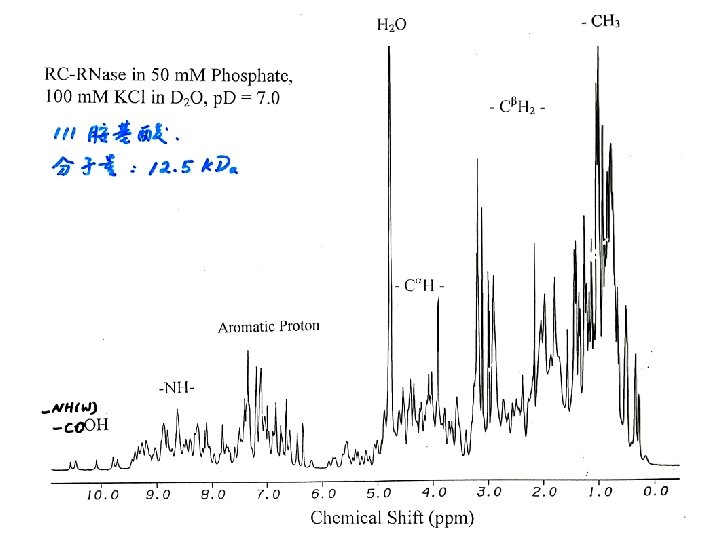
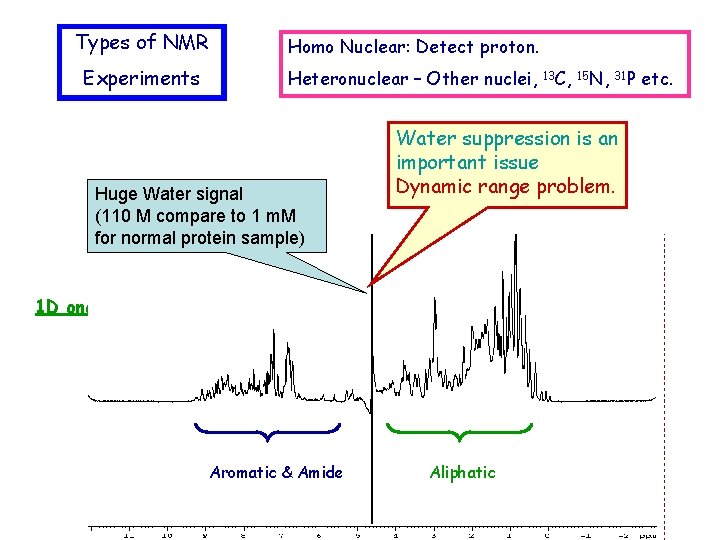
Types of NMR Homo Nuclear: Detect proton. Experiments Heteronuclear – Other nuclei, Huge Water signal (110 M compare to 1 m. M for normal protein sample) Water suppression is an important issue Dynamic range problem. 1 D one pulse 1 H Aromatic & Amide 13 C, 15 N, 31 P Aliphatic etc.

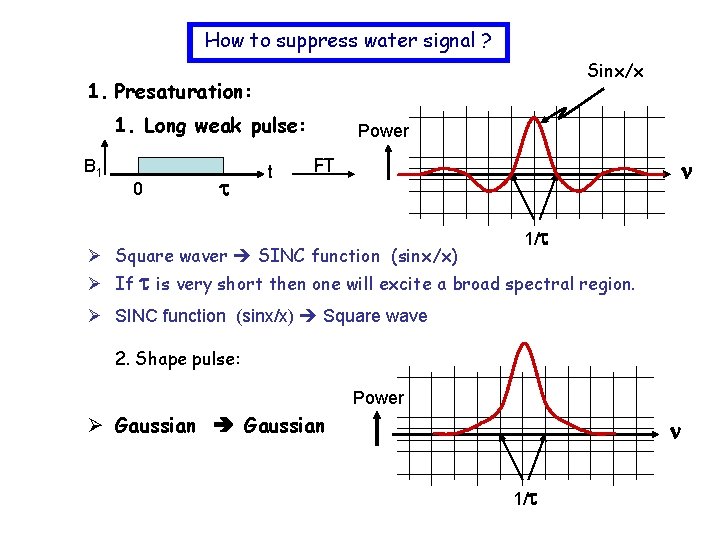
How to suppress water signal ? Sinx/x 1. Presaturation: 1. Long weak pulse: B 1 0 t Power FT Ø Square waver SINC function (sinx/x) 1/ Ø If is very short then one will excite a broad spectral region. Ø SINC function (sinx/x) Square wave 2. Shape pulse: Power Ø Gaussian 1/

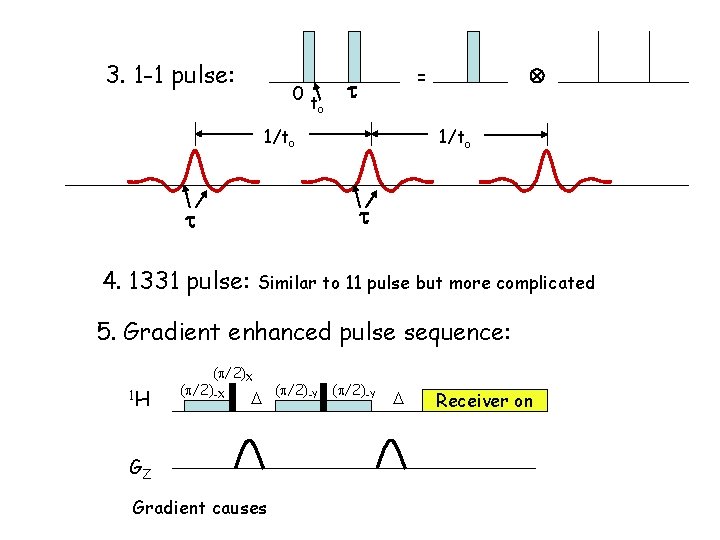
3. 1 -1 pulse: 0 t o = 1/to 4. 1331 pulse: Similar to 11 pulse but more complicated 5. Gradient enhanced pulse sequence: 1 H ( /2)X ( /2)-X GZ Gradient causes ( /2)-Y Receiver on

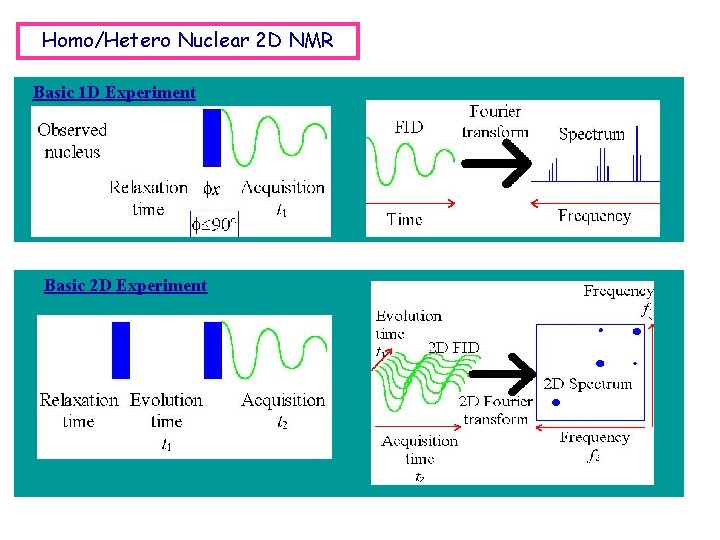
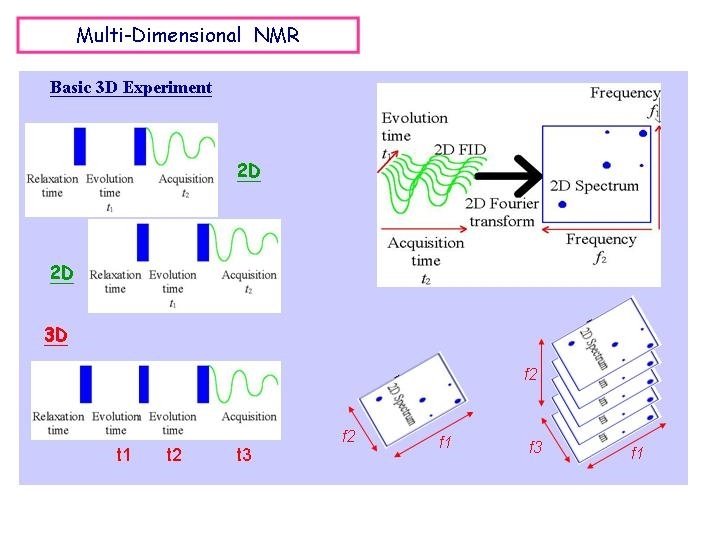
Homo/Hetero Nuclear 2 D NMR Basic 1 D Experiment Basic 2 D Experiment


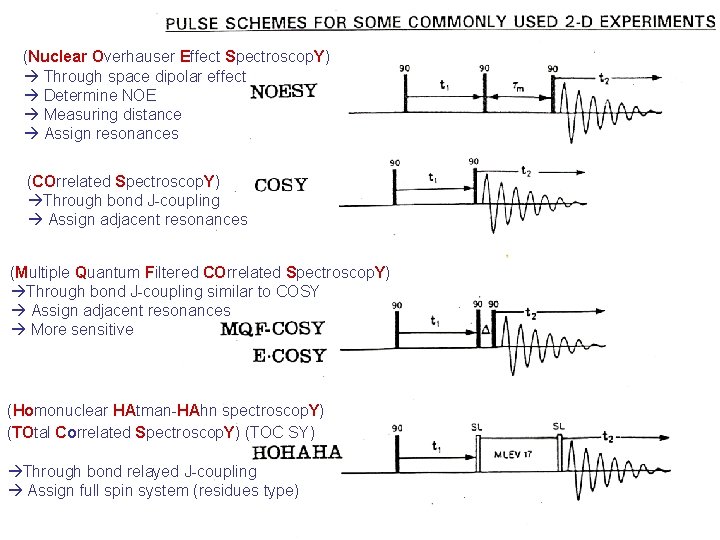
(Nuclear Overhauser Effect Spectroscop. Y) Through space dipolar effect Determine NOE Measuring distance Assign resonances (COrrelated Spectroscop. Y) Through bond J-coupling Assign adjacent resonances (Multiple Quantum Filtered COrrelated Spectroscop. Y) Through bond J-coupling similar to COSY Assign adjacent resonances More sensitive (Homonuclear HAtman-HAhn spectroscop. Y) (TOtal Correlated Spectroscop. Y) (TOC SY) Through bond relayed J-coupling Assign full spin system (residues type)

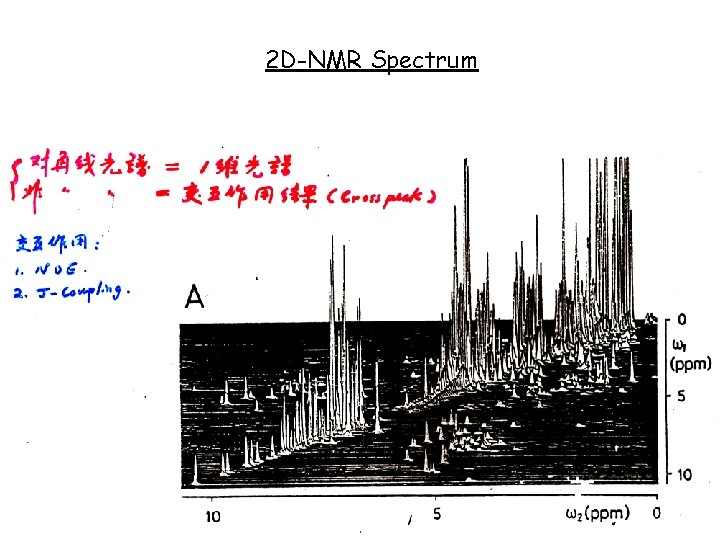
2 D-NMR Spectrum

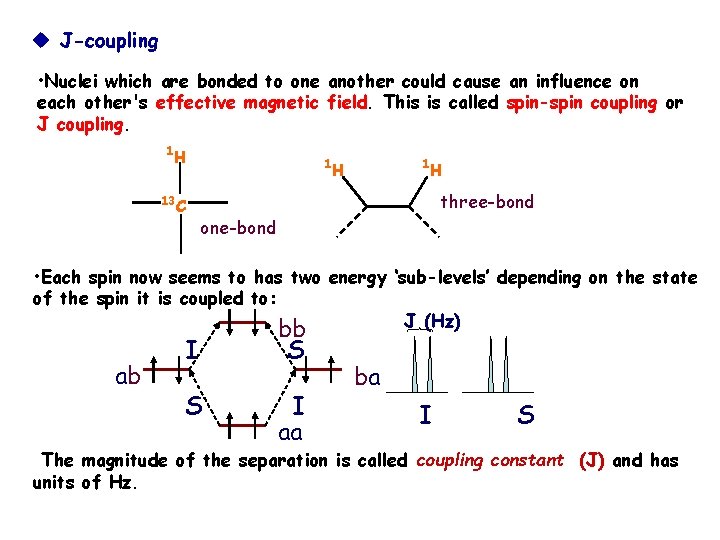
J-coupling • Nuclei which are bonded to one another could cause an influence on each other's effective magnetic field. This is called spin-spin coupling or J coupling. 1 H 13 1 1 H H three-bond C one-bond • Each spin now seems to has two energy ‘sub-levels’ depending on the state of the spin it is coupled to: J (Hz) ab I S bb S I aa ba I S The magnitude of the separation is called coupling constant (J) and has units of Hz.

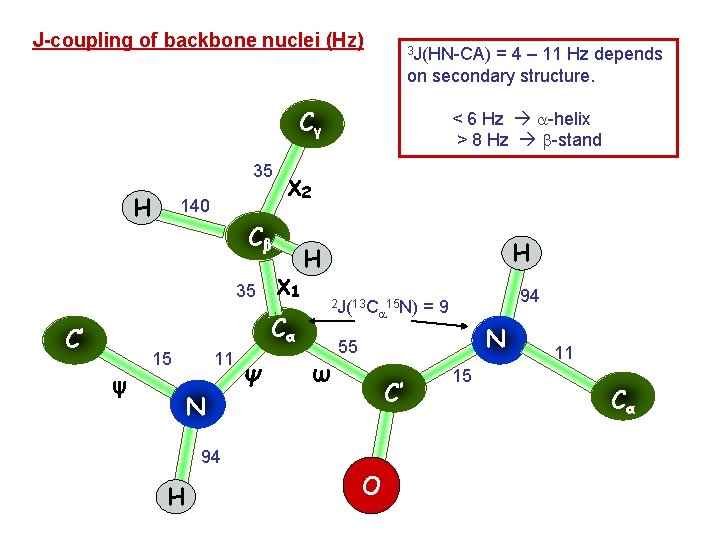
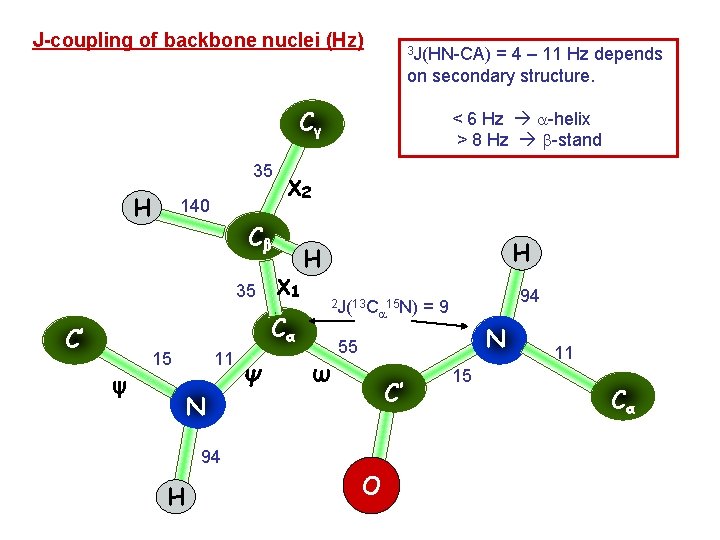
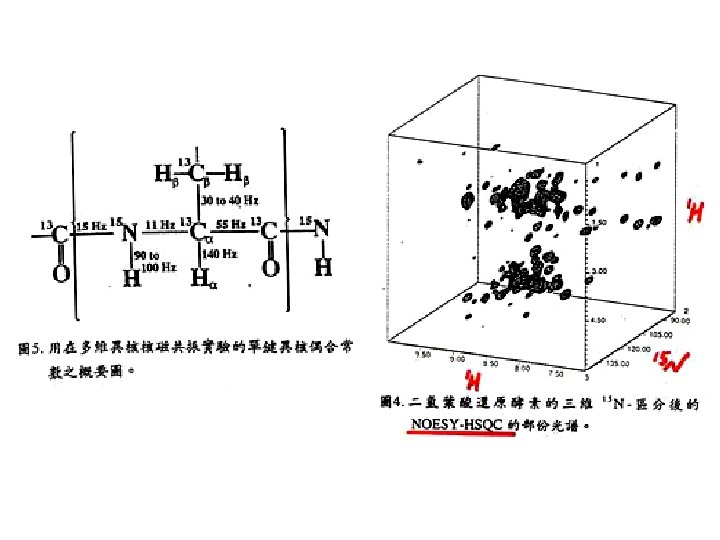
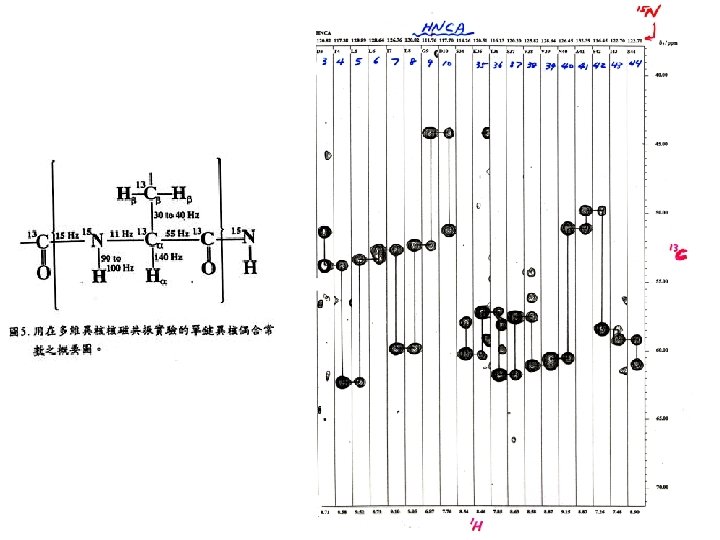
J-coupling of backbone nuclei (Hz) 3 J(HN-CA) = 4 – 11 Hz depends on secondary structure. Cγ 35 H 140 χ2 Cβ 35 χ1 Cα C’ ψ 15 11 N Ψ < 6 Hz -helix > 8 Hz -stand H H 2 J(13 C 15 N) ω N 55 C’ 94 H 94 =9 O 15 11 Cα

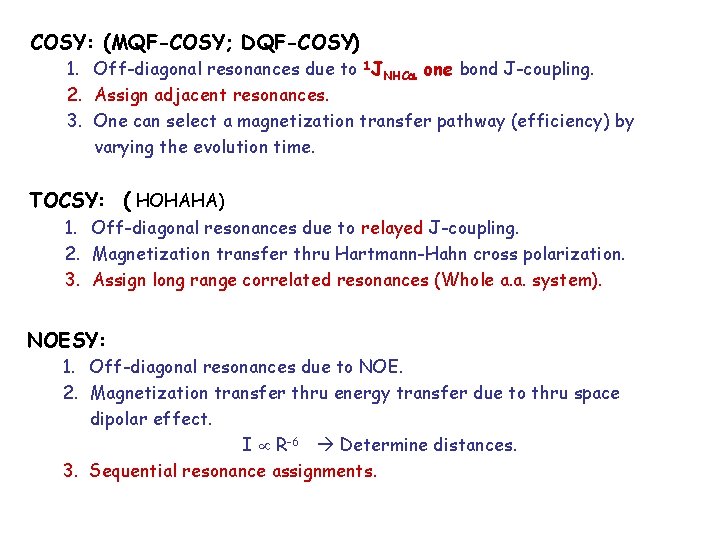
COSY: (MQF-COSY; DQF-COSY) 1. Off-diagonal resonances due to 1 JNHC one bond J-coupling. 2. Assign adjacent resonances. 3. One can select a magnetization transfer pathway (efficiency) by varying the evolution time. TOCSY: ( HOHAHA) 1. Off-diagonal resonances due to relayed J-coupling. 2. Magnetization transfer thru Hartmann-Hahn cross polarization. 3. Assign long range correlated resonances (Whole a. a. system). NOESY: 1. Off-diagonal resonances due to NOE. 2. Magnetization transfer thru energy transfer due to thru space dipolar effect. I R-6 Determine distances. 3. Sequential resonance assignments.

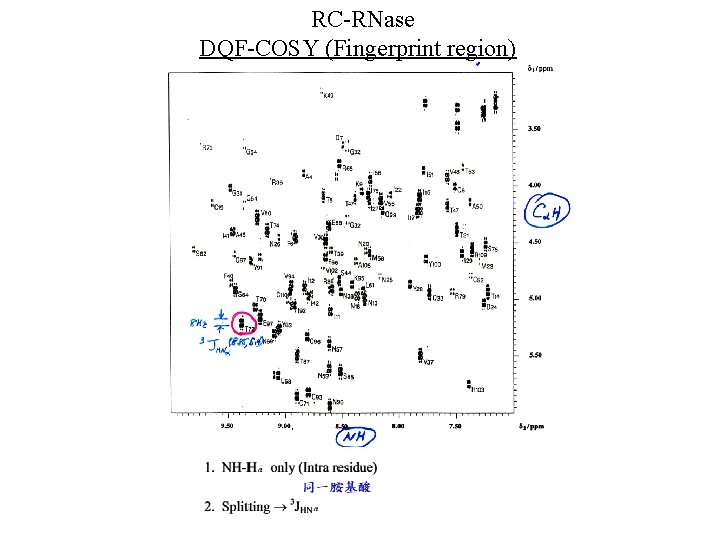
RC-RNase DQF-COSY (Fingerprint region)

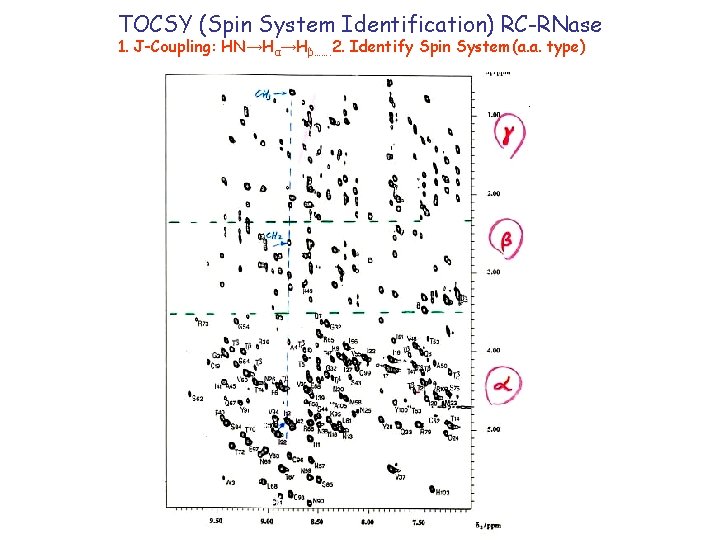
TOCSY (Spin System Identification) RC-RNase 1. J-Coupling: HN→Hα→Hβ……. 2. Identify Spin System(a. a. type) δ 1/ppm


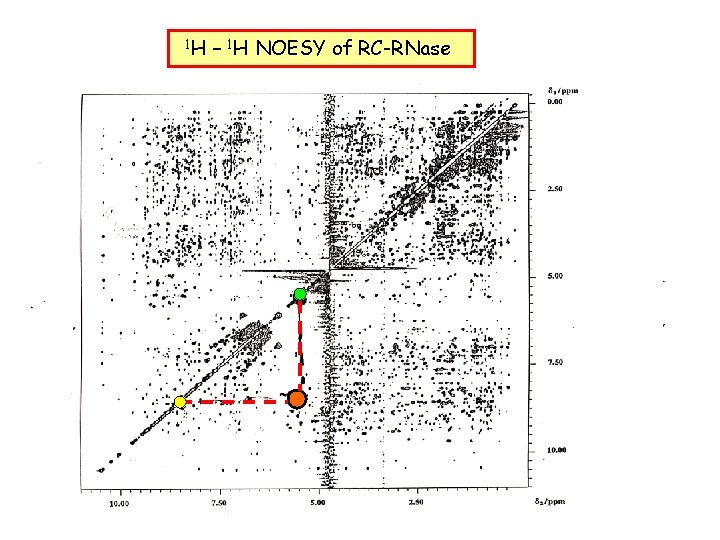
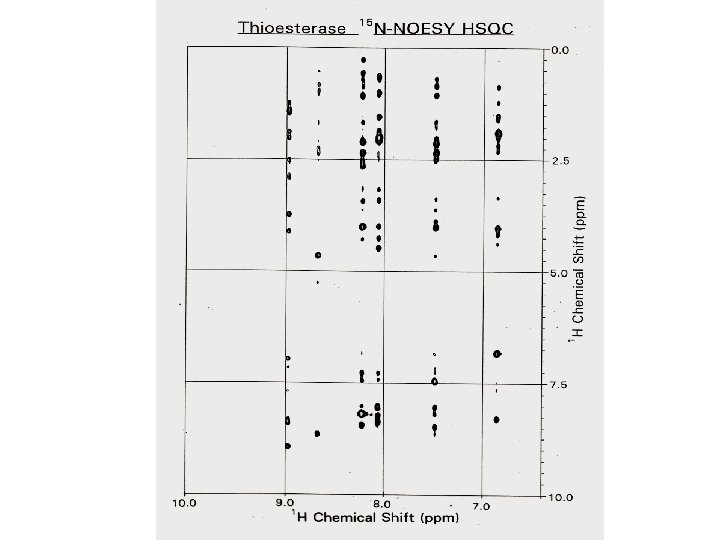
1 H – 1 H NOESY of RC-RNase

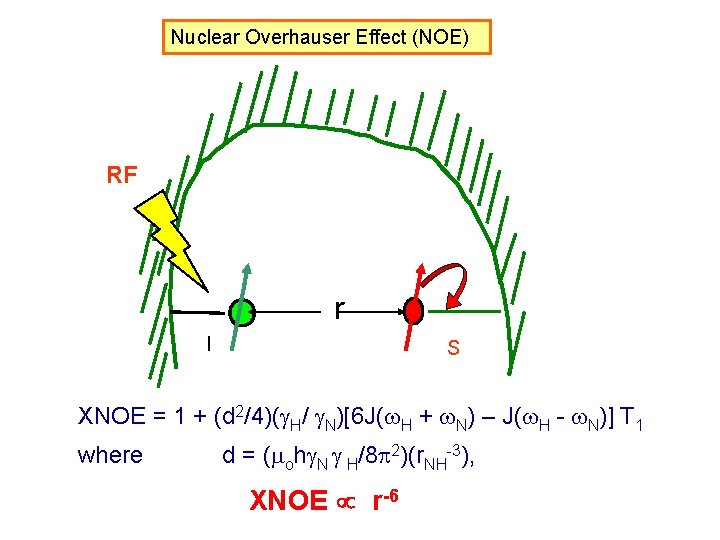
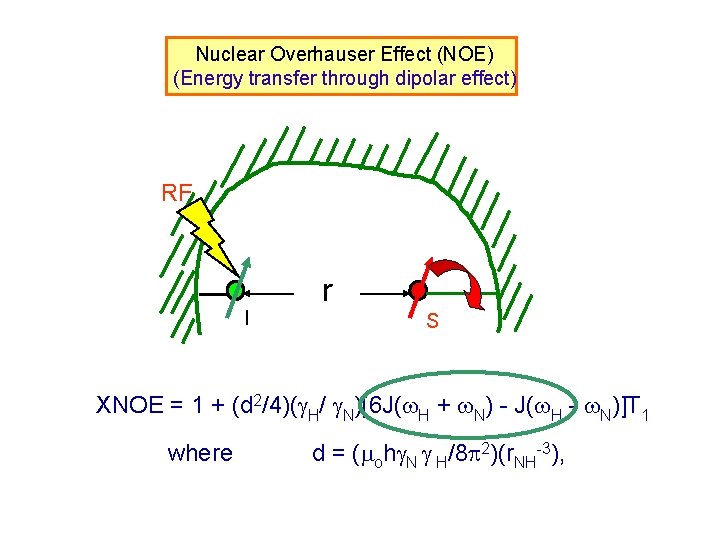
Nuclear Overhauser Effect (NOE) RF r I S XNOE = 1 + (d 2/4)( H/ N)[6 J( H + N) – J( H - N)] T 1 where d = ( oh N H/8 2)(r. NH-3), XNOE r-6


J-coupling of backbone nuclei (Hz) 3 J(HN-CA) = 4 – 11 Hz depends on secondary structure. Cγ 35 H 140 χ2 Cβ 35 χ1 Cα C’ ψ 15 11 N Ψ < 6 Hz -helix > 8 Hz -stand H H 2 J(13 C 15 N) ω N 55 C’ 94 H 94 =9 O 15 11 Cα

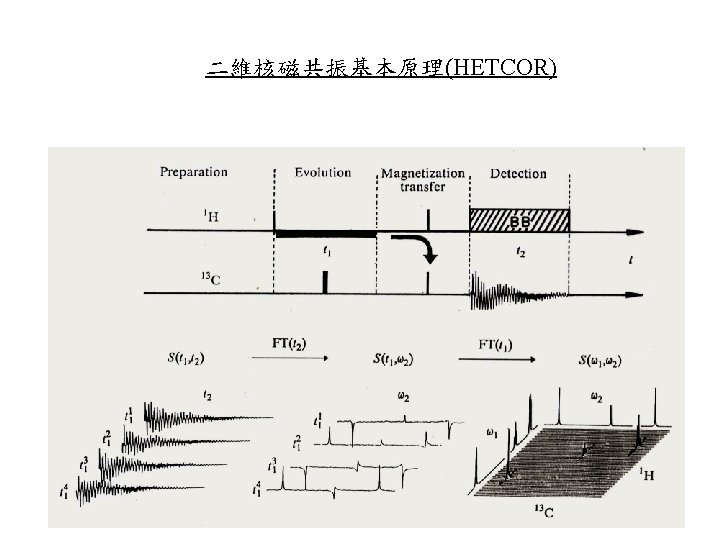
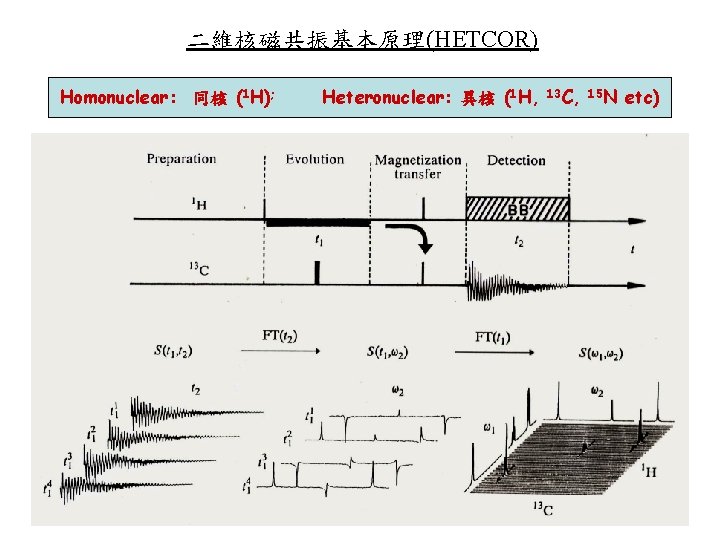
二維核磁共振基本原理(HETCOR) Homonuclear: 同核 (1 H); Heteronuclear: 異核 (1 H, 13 C, 15 N etc)

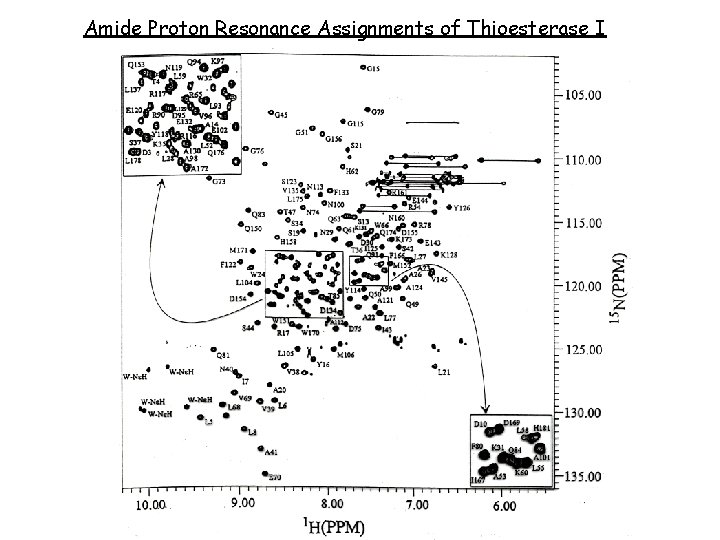
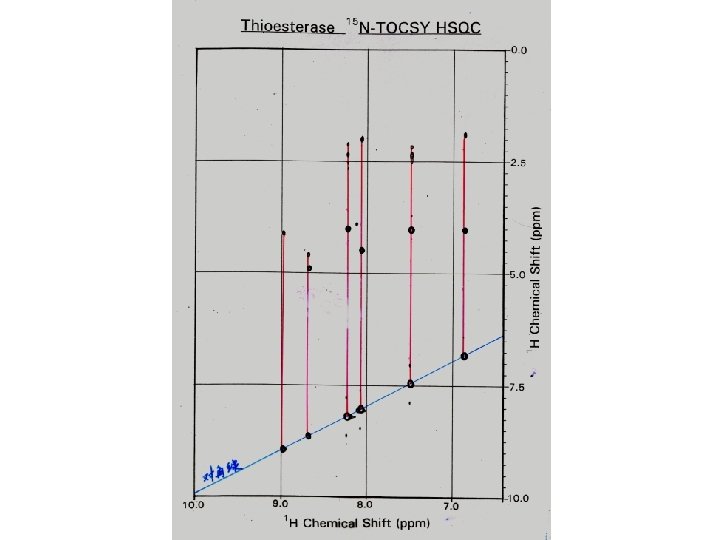
Amide Proton Resonance Assignments of Thioesterase I

Advantages of heteronuclear NMR: 1. 2. 3. 4. 5. Large chemical shift dispersion Increased resolution. Large coupling constant (Easy to transfer magnetization. Thru bond connectivity Easy assignments. Permit easier analysis of protein dynamics. Permit determining the structure of larger proteins (> 100 k. Da). Disadvantages of heteronuclear NMR: 1. Must label the protein with 13 C and/or 15 N. a). Expensive. b). Time consuming. 2. Technically much more complicated. 3. More demanding on spectrometers. 4. Much larger data size.





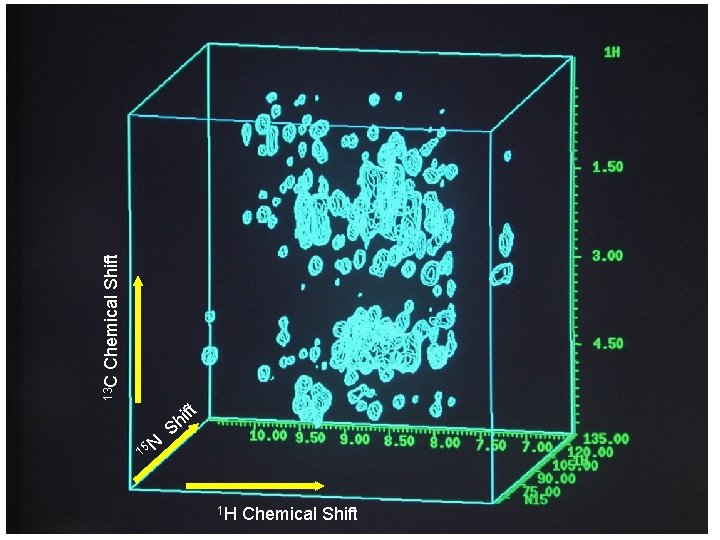
Chemical Shift 13 C ft 15 N i Sh 1 H Chemical Shift




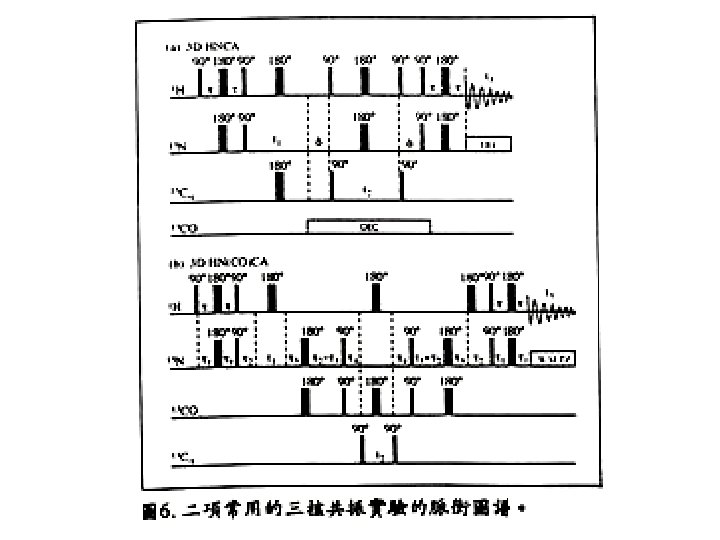
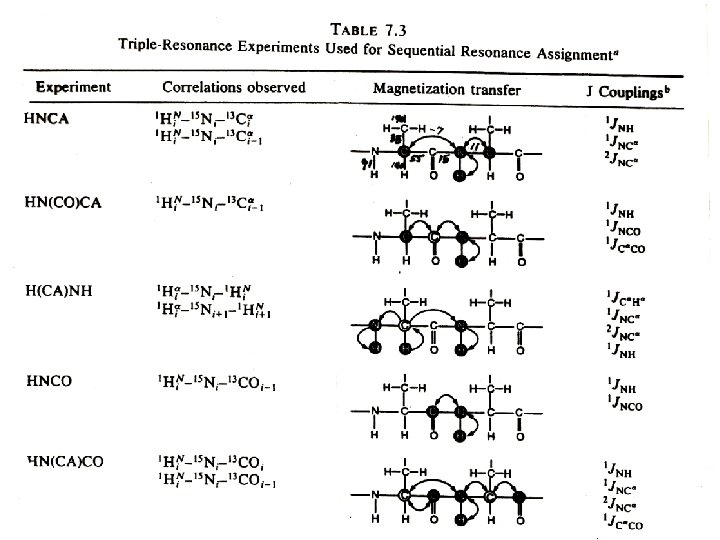
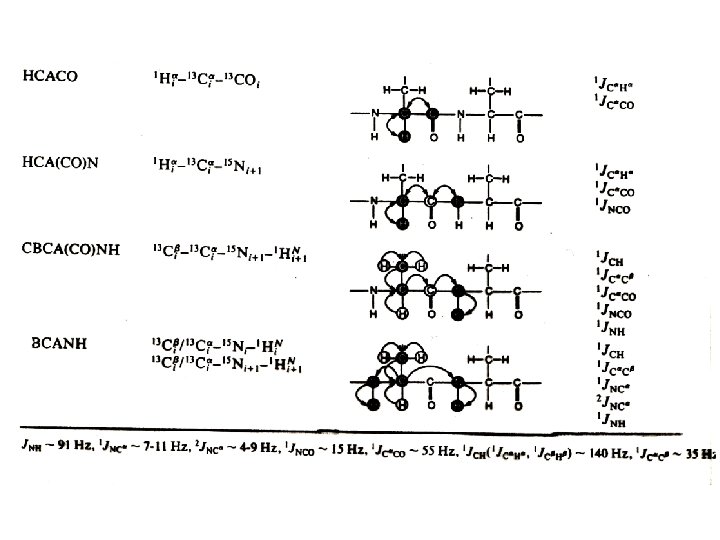
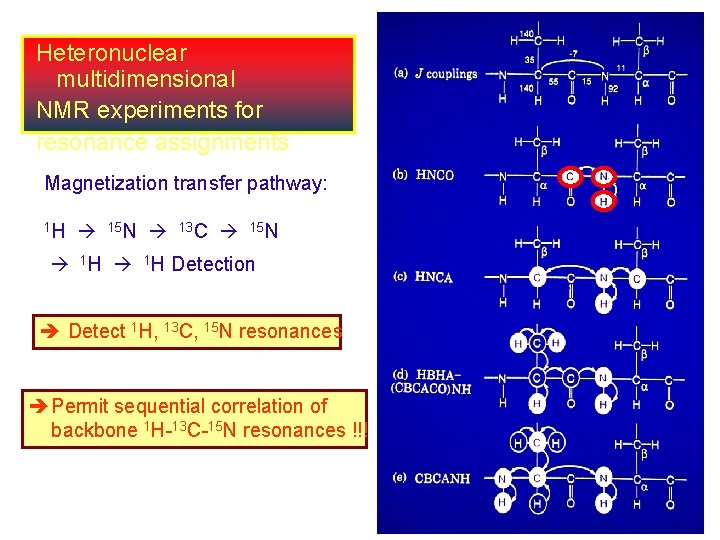
Heteronuclear multidimensional NMR experiments for resonance assignments Magnetization transfer pathway: 1 H 15 N 13 C 15 N 1 H Detection Detect 1 H, 13 C, 15 N resonances Permit sequential correlation of backbone 1 H-13 C-15 N resonances !!!


II. Dynamics 4 -dimensional structure

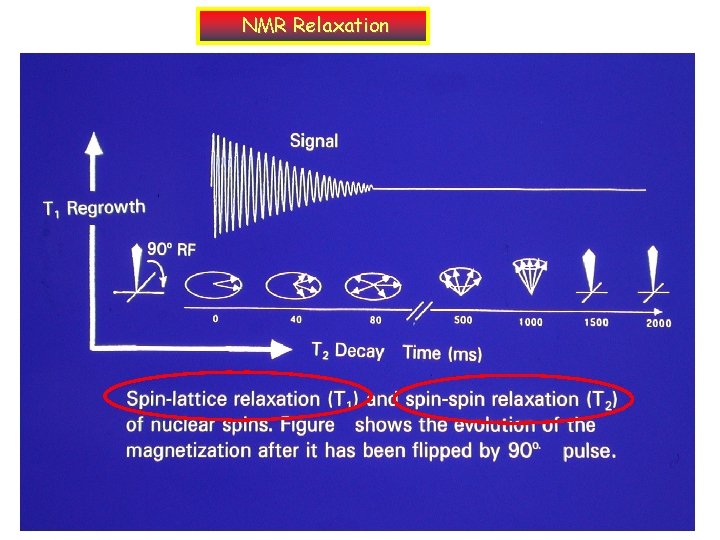
NMR Relaxation

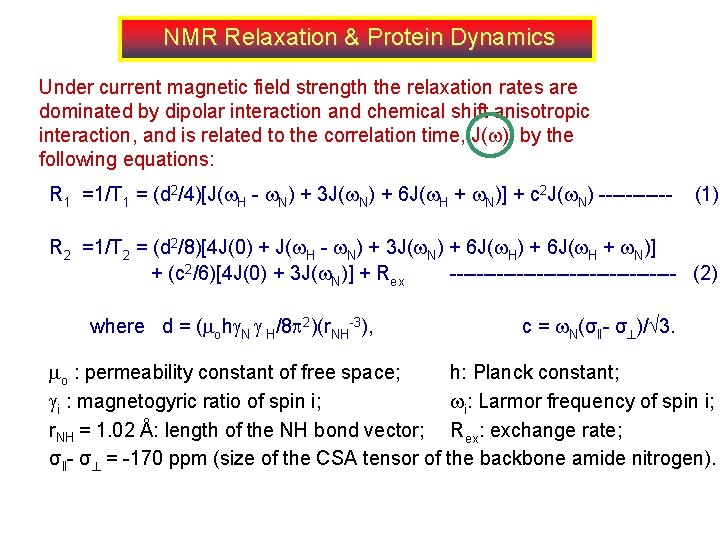
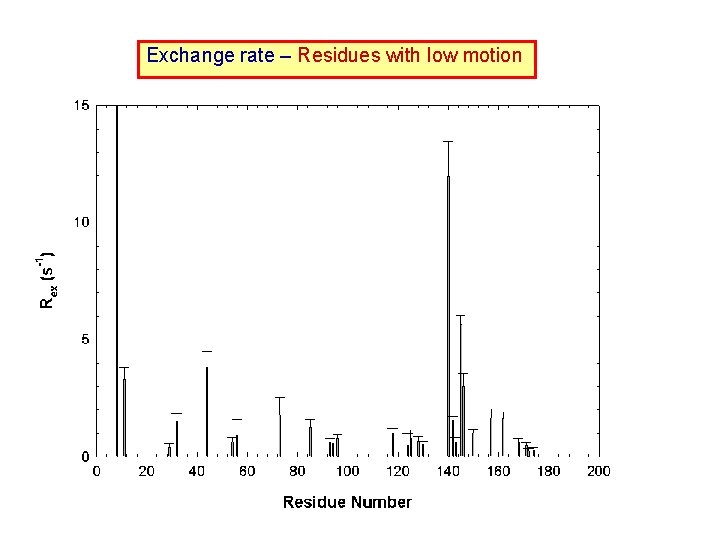
NMR Relaxation & Protein Dynamics Under current magnetic field strength the relaxation rates are dominated by dipolar interaction and chemical shift anisotropic interaction, and is related to the correlation time, J( ), by the following equations: R 1 =1/T 1 = (d 2/4)[J( H - N) + 3 J( N) + 6 J( H + N)] + c 2 J( N) ------ (1) R 2 =1/T 2 = (d 2/8)[4 J(0) + J( H - N) + 3 J( N) + 6 J( H + N)] + (c 2/6)[4 J(0) + 3 J( N)] + Rex ----------------- (2) where d = ( oh N H/8 2)(r. NH-3), c = N(σ‖- σ )/ 3. o : permeability constant of free space; h: Planck constant; i : magnetogyric ratio of spin i; i: Larmor frequency of spin i; r. NH = 1. 02 Å: length of the NH bond vector; Rex: exchange rate; σ‖- σ = -170 ppm (size of the CSA tensor of the backbone amide nitrogen).

Nuclear Overhauser Effect (NOE) (Energy transfer through dipolar effect) RF I r S XNOE = 1 + (d 2/4)( H/ N)[6 J( H + N) - J( H - N)]T 1 where d = ( oh N H/8 2)(r. NH-3),

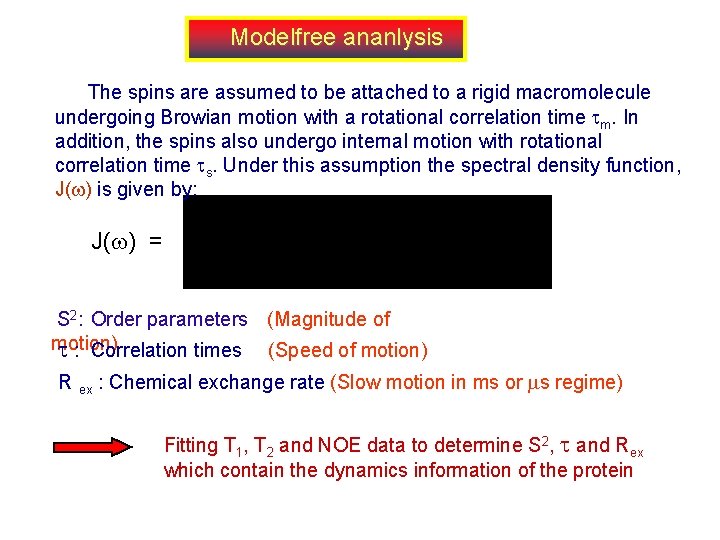
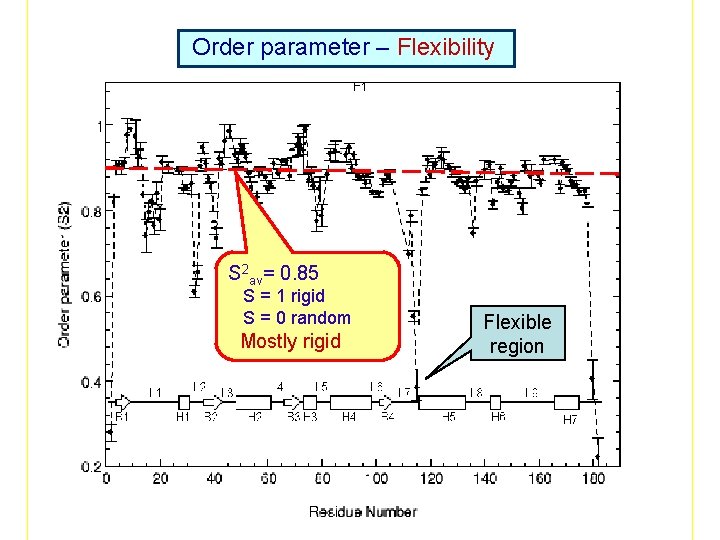
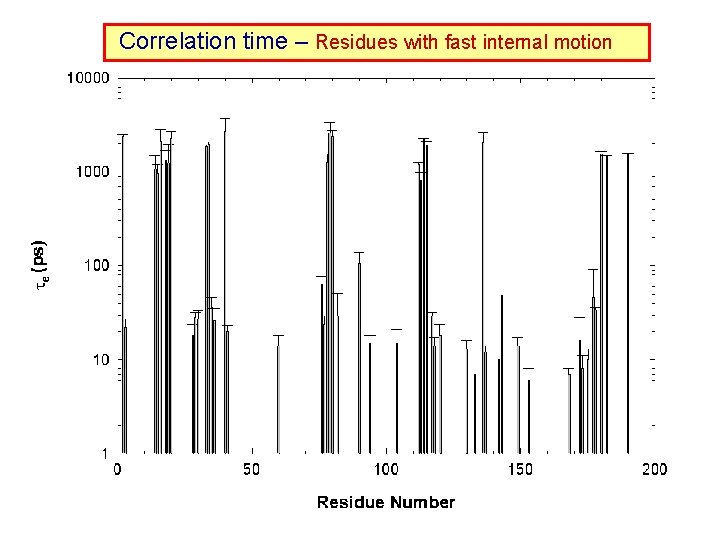
Modelfree ananlysis The spins are assumed to be attached to a rigid macromolecule undergoing Browian motion with a rotational correlation time m. In addition, the spins also undergo internal motion with rotational correlation time s. Under this assumption the spectral density function, J( ) is given by: J( ) = S 2: Order parameters (Magnitude of motion) : Correlation times (Speed of motion) R ex : Chemical exchange rate (Slow motion in ms or s regime) Fitting T 1, T 2 and NOE data to determine S 2, and Rex which contain the dynamics information of the protein

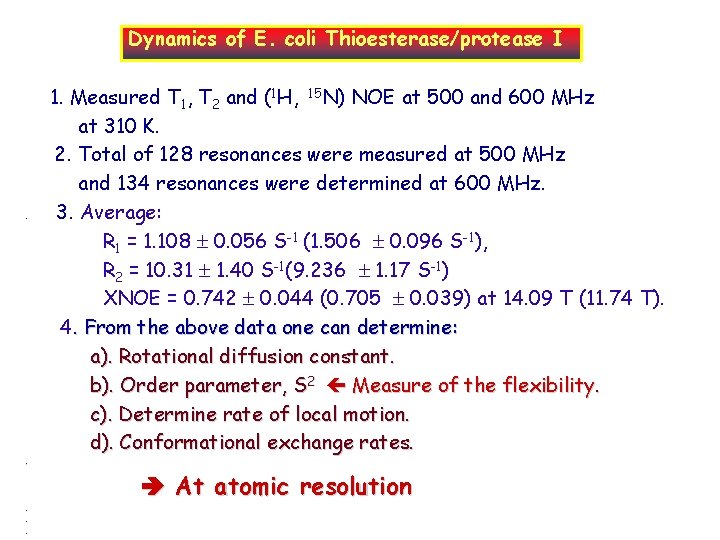
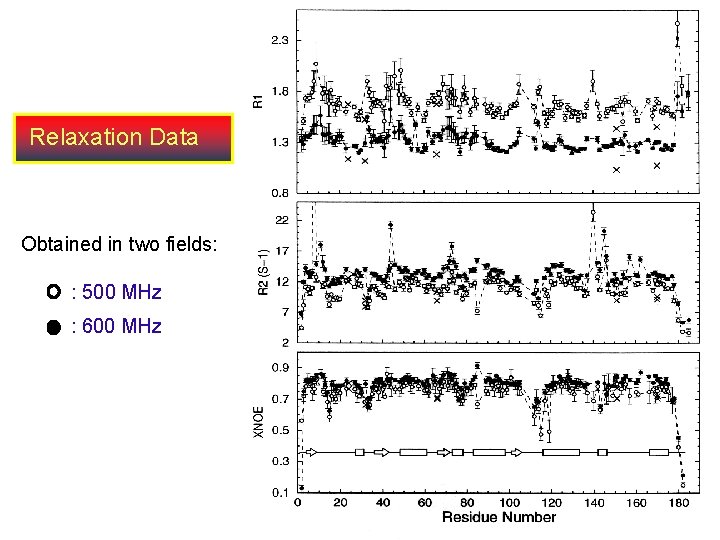
Dynamics of E. coli Thioesterase/protease I . . . 1. Measured T 1, T 2 and (1 H, 15 N) NOE at 500 and 600 MHz at 310 K. 2. Total of 128 resonances were measured at 500 MHz and 134 resonances were determined at 600 MHz. 3. Average: R 1 = 1. 108 0. 056 S-1 (1. 506 0. 096 S-1), R 2 = 10. 31 1. 40 S-1(9. 236 1. 17 S-1) XNOE = 0. 742 0. 044 (0. 705 0. 039) at 14. 09 T (11. 74 T). 4. From the above data one can determine: a). Rotational diffusion constant. b). Order parameter, S 2 Measure of the flexibility. c). Determine rate of local motion. d). Conformational exchange rates. At atomic resolution


Relaxation Data Obtained in two fields: : 500 MHz : 600 MHz

Order parameter – Flexibility S 2 av= 0. 85 S = 1 rigid S = 0 random Mostly rigid Flexible region

Correlation time – Residues with fast internal motion

Exchange rate – Residues with low motion

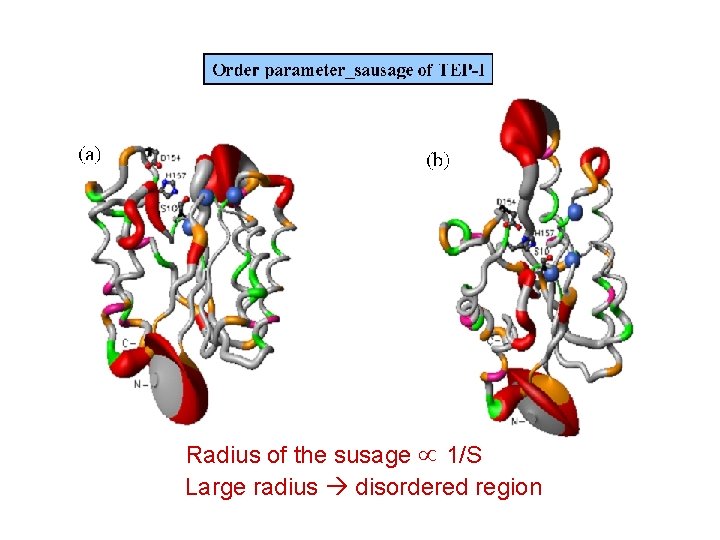
Radius of the susage 1/S Large radius disordered region

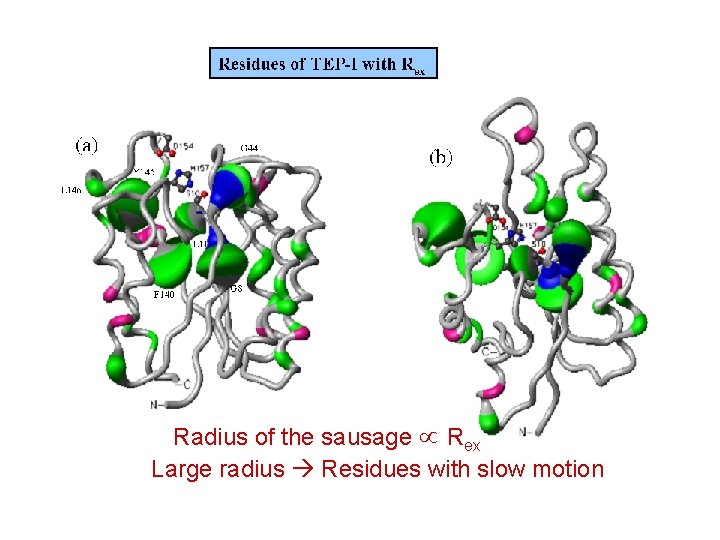
Radius of the sausage Rex Large radius Residues with slow motion

Thank You ! 800 MHz
