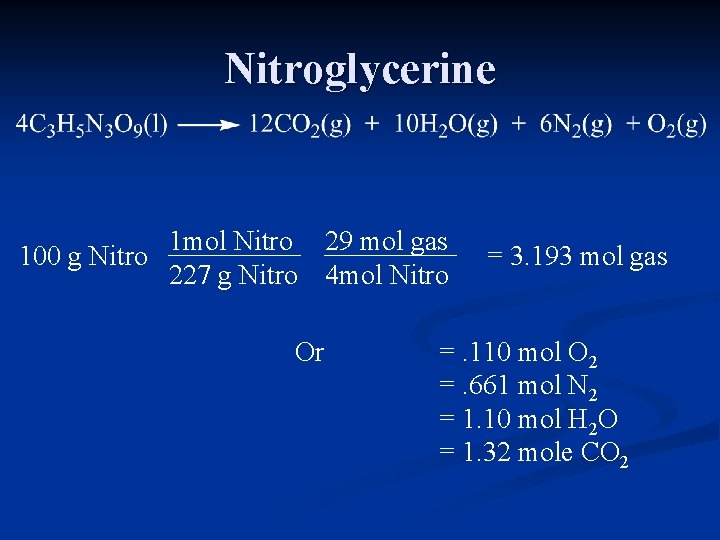
Nitroglycerine 1 mol Nitro 29 mol gas 100




- Slides: 14

Nitroglycerine 1 mol Nitro 29 mol gas 100 g Nitro 227 g Nitro 4 mol Nitro Or = 3. 193 mol gas =. 110 mol O 2 =. 661 mol N 2 = 1. 10 mol H 2 O = 1. 32 mole CO 2

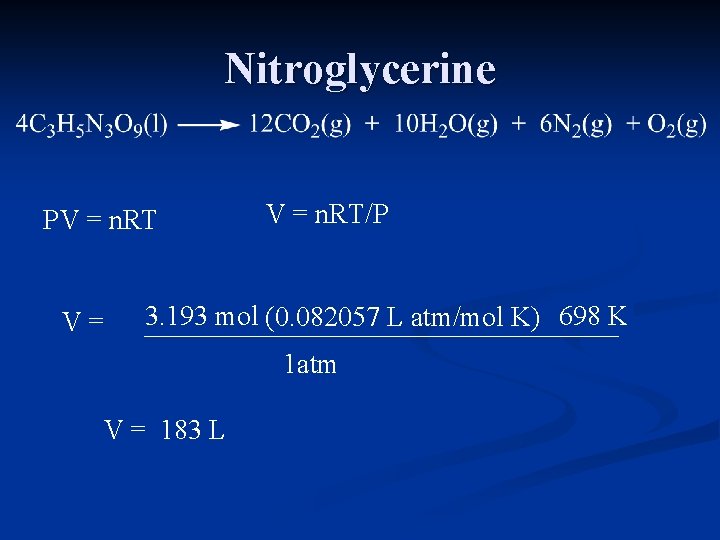
Nitroglycerine PV = n. RT V= V = n. RT/P 3. 193 mol (0. 082057 L atm/mol K) 698 425 K C 1 atm V = 183 L

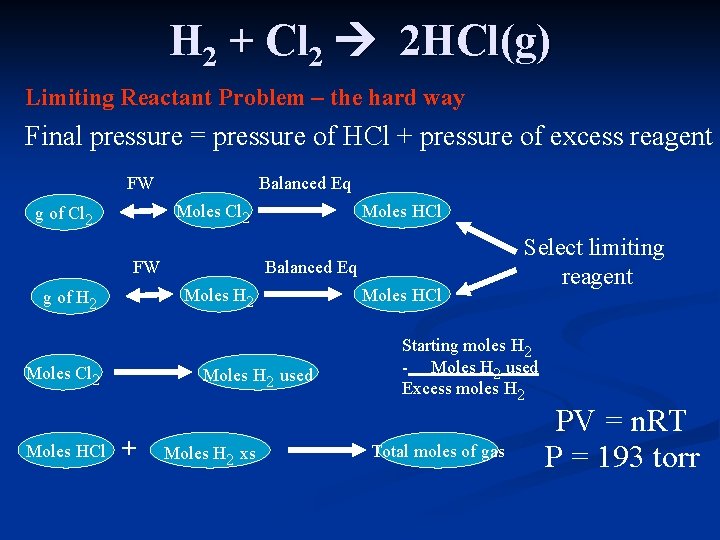
H 2 + Cl 2 2 HCl(g) Limiting Reactant Problem – the hard way Final pressure = pressure of HCl + pressure of excess reagent FW Balanced Eq Moles Cl 2 g of Cl 2 FW Balanced Eq Moles H 2 g of H 2 Moles Cl 2 Moles HCl Moles H 2 used + Moles H 2 xs Moles HCl Select limiting reagent Starting moles H 2 - Moles H 2 used Excess moles H 2 Total moles of gas PV = n. RT P = 193 torr

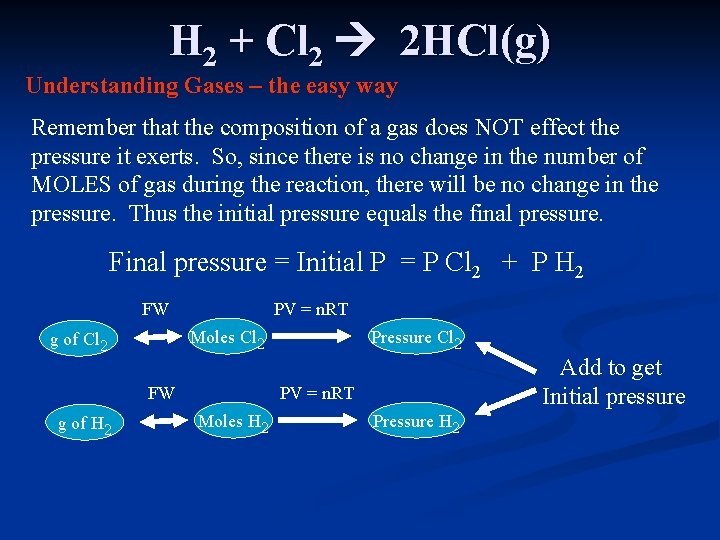
H 2 + Cl 2 2 HCl(g) Understanding Gases – the easy way Remember that the composition of a gas does NOT effect the pressure it exerts. So, since there is no change in the number of MOLES of gas during the reaction, there will be no change in the pressure. Thus the initial pressure equals the final pressure. Final pressure = Initial P = P Cl 2 + P H 2 FW PV = n. RT Moles Cl 2 g of Cl 2 FW g of H 2 Pressure Cl 2 Add to get Initial pressure PV = n. RT Moles H 2 Pressure H 2

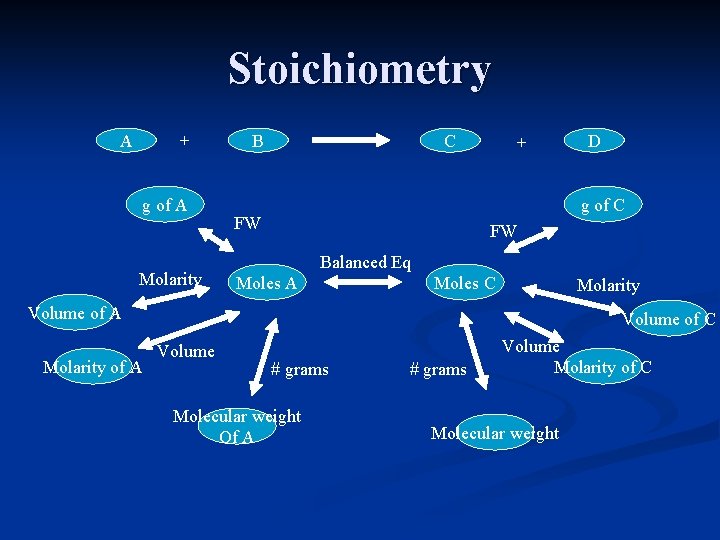
Stoichiometry A + g of A Molarity B C + g of C FW FW Balanced Eq Moles A Moles C Molarity Volume of A Molarity of A D Volume of C Volume # grams Molecular weight Of A # grams Volume Molarity of C Molecular weight

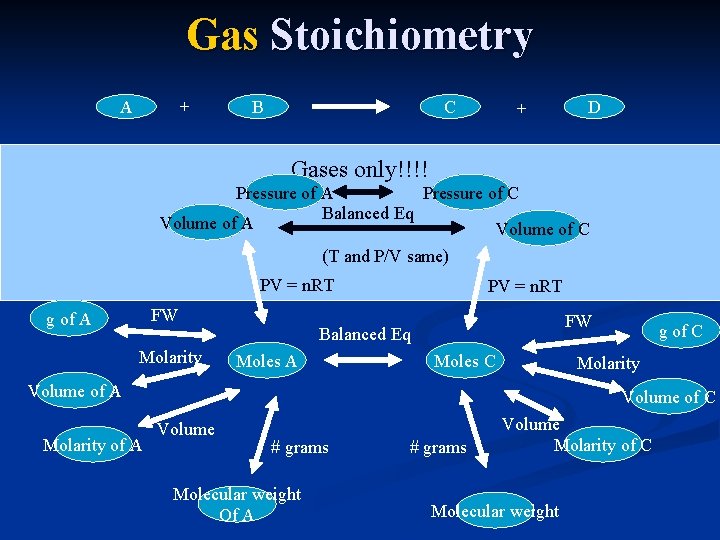
Gas Stoichiometry A + B C + D Gases only!!!! Pressure of A Pressure of C Balanced Eq Volume of A Volume of C (T and P/V same) PV = n. RT FW g of A Molarity PV = n. RT FW Balanced Eq Moles A Moles C Molarity Volume of A Molarity of A g of C Volume # grams Molecular weight Of A # grams Volume Molarity of C Molecular weight

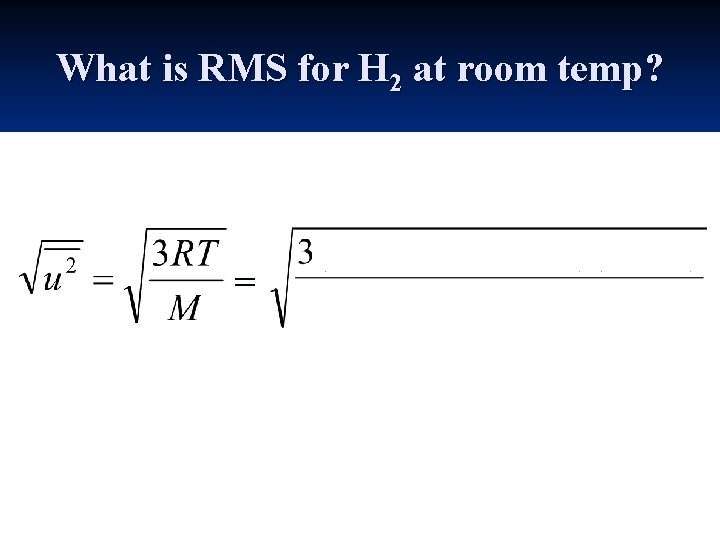
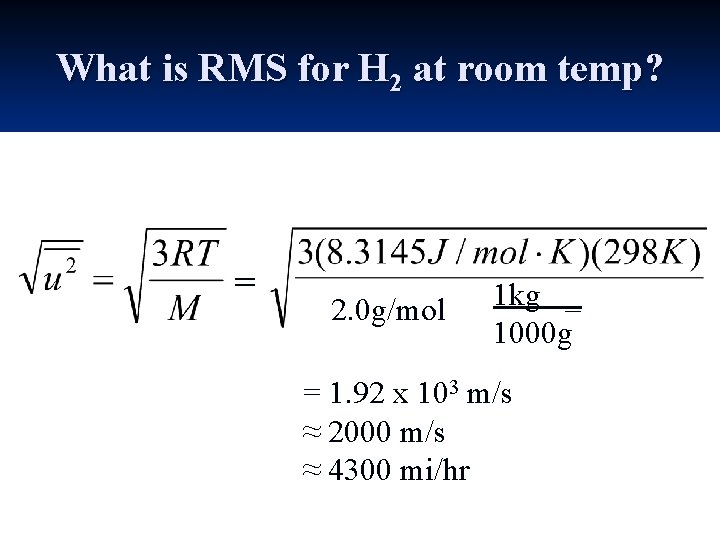
What is RMS for H 2 at room temp? =

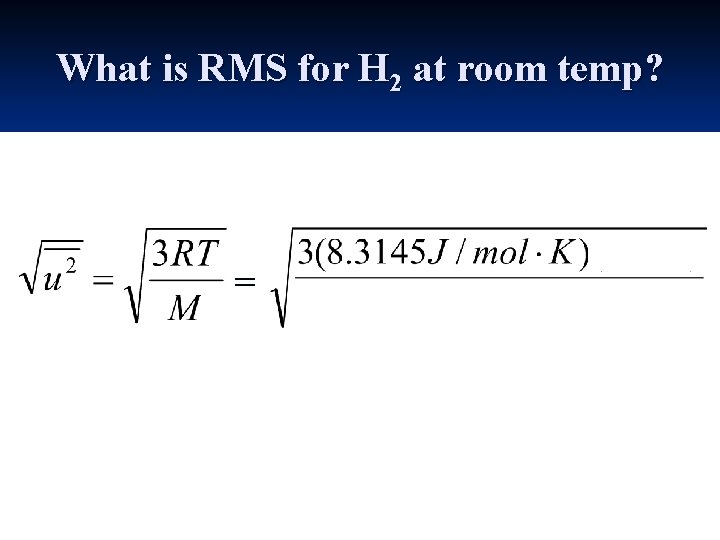
What is RMS for H 2 at room temp? =

What is RMS for H 2 at room temp? = 2. 0 g/mol 1 kg _ 1000 g = 1. 92 x 103 m/s ≈ 2000 m/s ≈ 4300 mi/hr

Why does it take so long to smell gas that is released in the same room?

Problems involving Gases n PV=n. RT can be used to get moles of gas from P, V and T. Once you have mole then it is just a normal limiting reagent or stoichiometry or titration or ….

Gas Law Shortcuts All revolve around the fact that in gases, V and P does NOT depend on nature of gas. n Implications n Can sum moles of all products and then find P or V. (Dalton’s Law of Partial Pressures. ) n Ratio of n: V or n: P is constant (at a given temp and P or V) So 2 moles of gas C takes up twice the volume of 1 mole of gas A at the same conditions. n n Caution: Shortcuts are NOT always present.

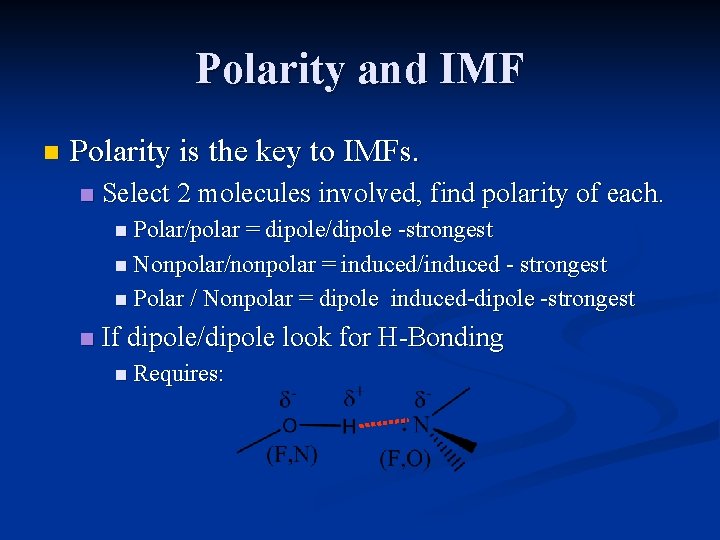
Polarity and IMF n Polarity is the key to IMFs. n Select 2 molecules involved, find polarity of each. n Polar/polar = dipole/dipole -strongest n Nonpolar/nonpolar = induced/induced - strongest n Polar / Nonpolar = dipole n induced-dipole -strongest If dipole/dipole look for H-Bonding n Requires:

Why don’t oil and water mix?