NITROGEN FAMILY GROUP 5 A Nitrogen is an






















- Slides: 31

NITROGEN FAMILY GROUP 5 A Nitrogen is an important element in agriculture (look in page 63 )


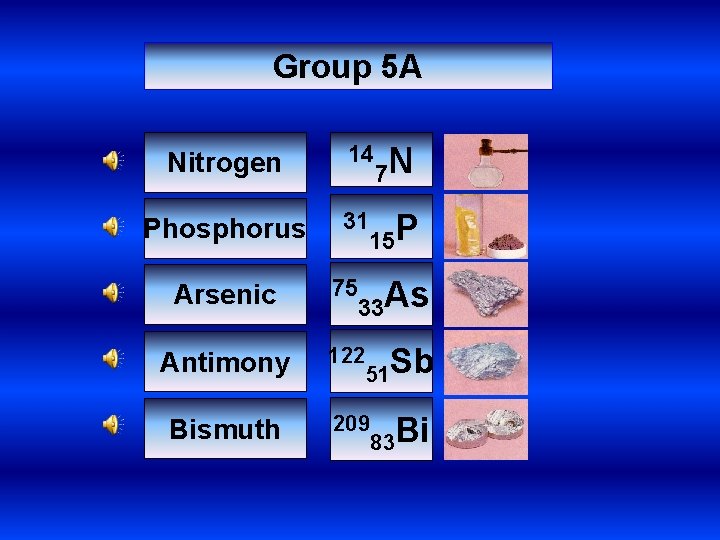
Group 5 A Nitrogen 14 Phosphorus 31 7 N 15 P 33 As Arsenic 75 Antimony 122 Bismuth 209 51 Sb 83 Bi

NITROGEN Family *Common oxidation numbers are all between- 3 and +5. *Metallic character increases from top to bottom. *N and P are nonmetals, As and Sb are metalloid, Bi is a metal. *All have allotropes, except nitrogen and bismuth.

General information *Other name is azot, which means dead in Latin language. *Nitrogen is a colorless, odorless, tasteless gas which is discovered in Scotland in 1772 by D. Rutherford. *Lighter than air. *The main mineral sources are KNO 3 and Na. NO 3, common name of Nitrates is potash.

General information about NITROGEN *Forms diatomic structure (N 2) and is an inactive element. *Nitrogen is very important for living organisms, exist in construction of proteins and nucleic acids. *Isotopes are 99. 64% 14 N and 0. 36% 15 N

General information about NITROGEN Boiling point: -195. 8 0 C Common oxidation num. : -3, +1, +2, +3, +4, +5

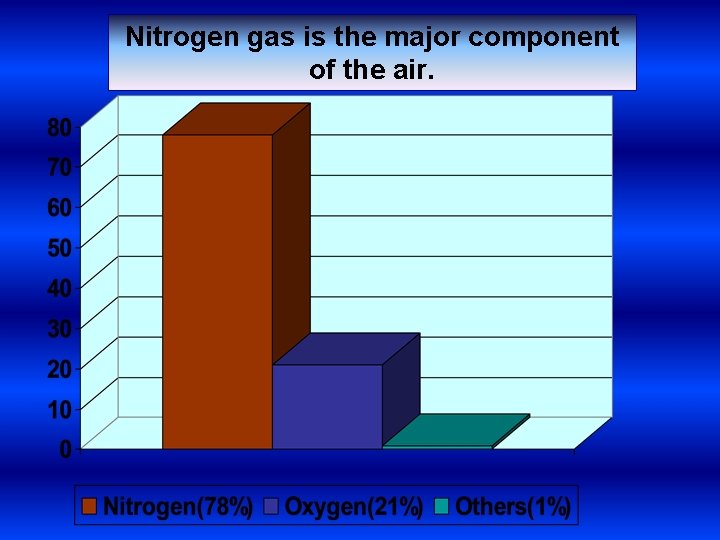
Nitrogen gas is the major component of the air.

Preparation The main source of N 2 is the air and it is obtained by fractional distillation of liquefied air in industry. Nitrogen evaporates first during the distillation.

Preparation 1. Na. NO 2 + NH 4 Cl Na. Cl + 2 H 2 O + N 2(g) 2. After removing CO 2 from air 2 Cu(s) + (3 N 2+O 2) 2 Cu. O(s) + 2 N 2(g)

Preparation 3. 2 Na. N 3(s) 2 Na(s) + 3 N 2(g) This reaction is applied in the air-bag of automobiles.

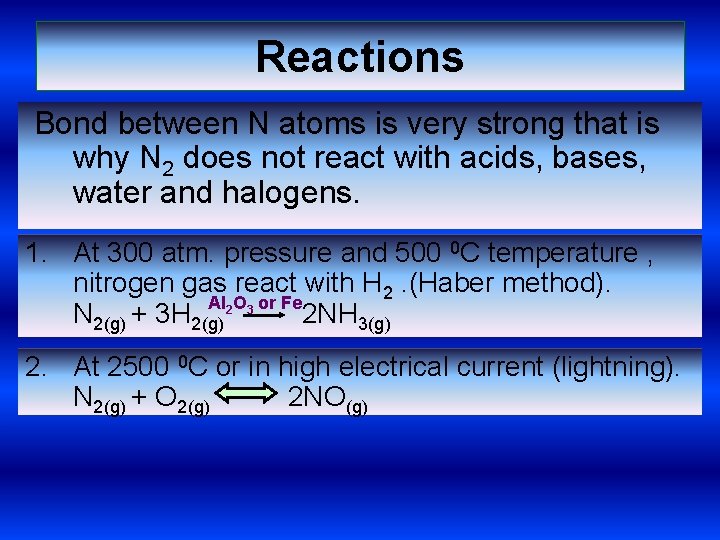
Reactions Bond between N atoms is very strong that is why N 2 does not react with acids, bases, water and halogens. 1. At 300 atm. pressure and 500 0 C temperature , nitrogen gas react with H 2. (Haber method). Al 2 O 3 or Fe N 2(g) + 3 H 2(g) 2 NH 3(g) 2. At 2500 0 C or in high electrical current (lightning). N 2(g) + O 2(g) 2 NO(g)

Compounds AMMONIA(NH 3): Colorless gas, has a sharp smell Lower density than air Has a very high solubility in water Ammonia-NH 3, is an important compound used in the production of fertilizers. It is used in commercial cleaning products. In 2006, worldwide production was estimated at 146. 5 million tons.

Preparation of NH 3 Preparation in Industry: Haber Process N 2(g) + 3 H 2(g) heat and pressure Al 2 O 3 2 NH 3(g) Haber, Fritz (1868 -1935), German chemist and Nobel laureate, best known for his development of an economical method of ammonia synthesis.

Oxides of Nitrogen The most important oxides are N 2 O, NO and NO 2. Dinitrogen monoxide, N 2 O: Common name is nitrous oxide or laughing gas. Used as a mild anesthetic by dentists. Little amount is exist in atmosphere. Prepared by decomposition of ammonium nitrate (dangerous). NH 4 NO 3(s) heat N 2 O(g)+ 2 H 2 O(g) Decomposition of N 2 O. 2 N 2 O(g) 2 N 2(g) +O 2(g) don’t do it okey, okey

Oxides of Nitrogen NO, Nitrogen monoxide, harmful gas NO gas is produced by lightning in a nitrogen and oxygen gas mixture. lightning N 2(g) + O 2(g) 2 NO(g) Ignition of car motors also produces NO gas. By reacting with oxygen may form NO 2 , when inhaled, NO 2 by reacting with water may form HNO 3 acid in lungs, 2 NO(g) + O 2(g) 2 NO 2(g) ignition lightning

Nitric Acid(HNO 3) A colorless liquid with 5. 2 g/ml of density. Melting point is -42 0 C and the boiling point is 82. 6 0 C. Formed when metallic nitrate heated with concentrated sulfuric acid. Na. NO 3(s) + H 2 SO 4(aq) Na. HSO 4(aq) + HNO 3(aq) Nitric acid is the third most important industrial acid (after sulfuric and phosphoric acid). It is used to prepare fertilizers, explosives, nylon and plastics. Explosives Plastics Fertilizers

Nitric Acid (HNO 3) Lightning may cause to form nitric acid. May be acid rain Do you afraid of rain Lightning may cause to form nitric acid.

Reactions of Nitric acid does not react with Pt and Au, but the mixture of 3 volume of HCl and 1 volume of HNO 3, which is called aqua regia, reacts with Pt and Au, and this is the only acid mixture which may affect Pt and Au.

Uses of N 2 *metallurgy *explosive *dye ammonia *paper *textile *medicine *fertilizers. *to keep frozen foods *production of ammonia *inert atmosphere *manufacture of hardware

TEST YOURSELF

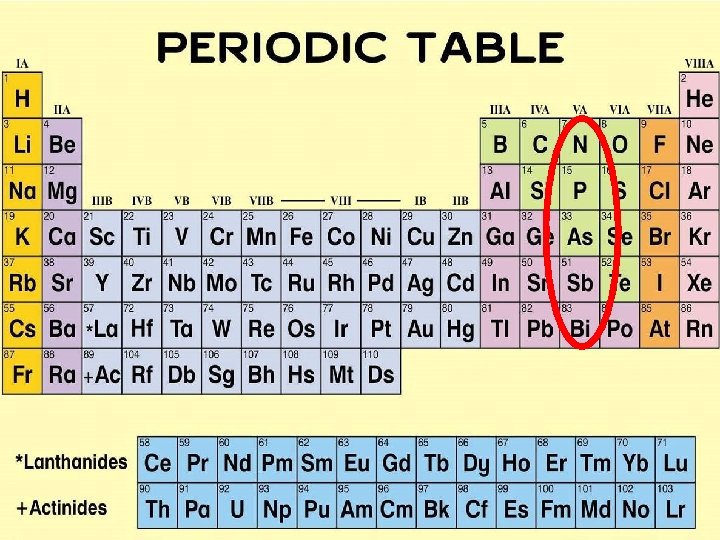
1. Which nonmetals are located in 5 A group?

2. What is another name of nitrogen which means dead in Latin language? ,

3. What is general name of nitrates of alkaline metals, which are used as fertilizers?

4. What is percentage of nitrogen in air, by volume?

5. How can we obtain pure nitrogen from air?

6. What can be said about the chemical activity of nitrogen gas?

7. What is the method that is used in industry to produce ammonia?

8. What is aqua regia?

9. Which compound of nitrogen can be produced during lightning?

10. What are the some uses of nitrogen?