Nitrogen Anl Sena BAYINDIR History of Nitrogen Daniel
Nitrogen Anıl Sena BAYINDIR

History of Nitrogen • Daniel Rutherford • 1772

History of Nitrogen • Lord Rayleigh, in 1910 • The Nitrogen is really reactive with many metals and many compounds.

What is a Nitrogen? • • Non-metal It has 7 electrons. Its nucleon number is 14(7 p, 7 n) “N”

What is Nitrogen? • Colorless • Odorless • Gas

What is Nitrogen? • It is found in the nature as N 2 molecules. (diatomic)
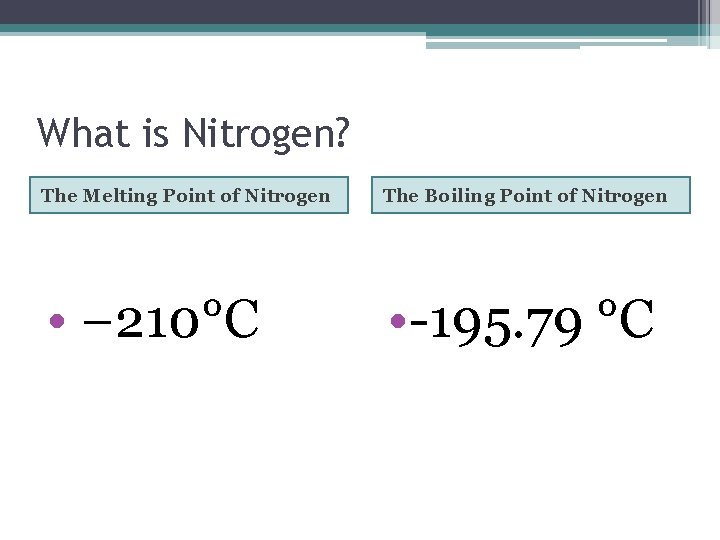
What is Nitrogen? The Melting Point of Nitrogen The Boiling Point of Nitrogen • − 210°C • -195. 79 °C

What is Nitrogen? • 78% of the air • The nitrogen inhaled is not used in our body. • is also taken with proteins(to build muscles, bones)

How to get Nitrogen ? • NH 4 Cl(aq) + Na. NO 2(aq) → N 2(g) + Na. Cl(aq) + 2 H 2 O (l • 2 Na. N 3 → 2 Na + 3 N 2

How can we use Nitrogen ? • Prevents things from reacting with oxygen • Fills some tires. • Liquid nitrogen can be used to freeze things.

Important!!! • It is poisonous. • So, while experimenting we should be careful.

EXPERIMENT TIME!!! “Explosion Of Ping Pong Balls with Liquid Nitrogen. ”

First Step • You should wear your glasses, your glasses and any other materials that will keep you safe for your safety. • Thanks for attention

Second Step • You will fill liquid nitrogen to a bottle until it is half with the help of funnel. • Small Reminder: Don’t try this at home

Third Step • You will close the mouth of the bottle with a cover and you will put it into a large container with 4000 ping pong balls.

Last Step • And, after you locate the balls and bottle into the container, you will go away from the container. • Then, you will see the brilliant result of this experiment

Thankk You !!!!
- Slides: 17