Nitrogen and Sulfur 18 Essential Elements Macronutrients Carbon

Nitrogen and Sulfur

18 Essential Elements Macronutrients: Carbon (C) Hydrogen (H) Oxygen (O) Nitrogen (N) Phosphorus (P) Potassium (K) Micronutrients: Iron (Fe) Manganese (Mn) Boron (B) Zinc (Zn) Copper (Cu) Chlorine (Cl) Calcium (Ca) Magnesium (Mg) Sulfur (S) Cobalt (Co) Molybednum (Mo) Nickel (Ni)

Plant Available Forms of Nitrogen NH 4+ Ammonium NO 3 - nitrate üWhich form is cation/anion? üWhich form held by clay and humus? üWhich form do plants prefer? üWhich form leaches?

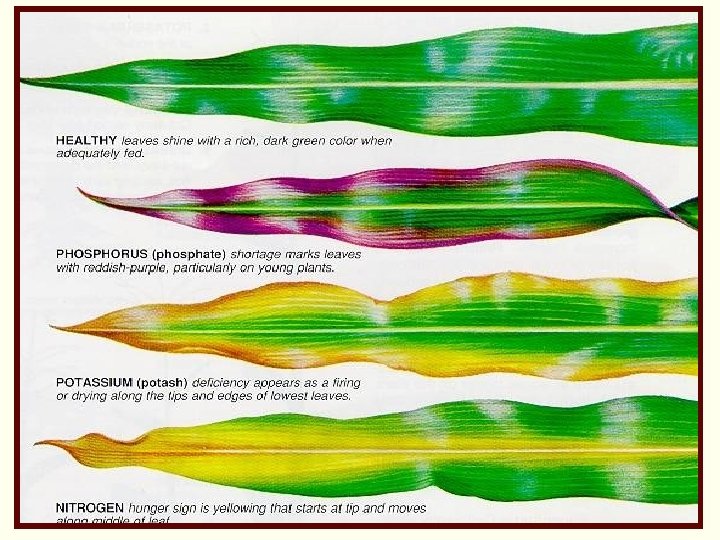
Importance of Nitrogen in Plant Nutrition ü Stimulates vegetative growth ü Gives deep green color to leaves ü Component of: 1. chlorophyll and enzymes 2. amino acids 3. nucleic acids


Too Much Nitrogen ü Encourages soft, weak, easily injured growth. ü May slow maturity and ripening of crops ü May delay “hardening-off” process that protects plants from winter cold. ü Plants may accumulate high levels of nitrate which can be toxic to cattle (sorghum spp. )

Nitrogen Deficiency in Corn Chlorosis (yellowing) at the tip and midrib of the oldest leaves.

Nitrogen Is Mobile in the Plant ü Because N is mobile in the plant… the deficiency symptoms will shop up first on OLDER leaves.
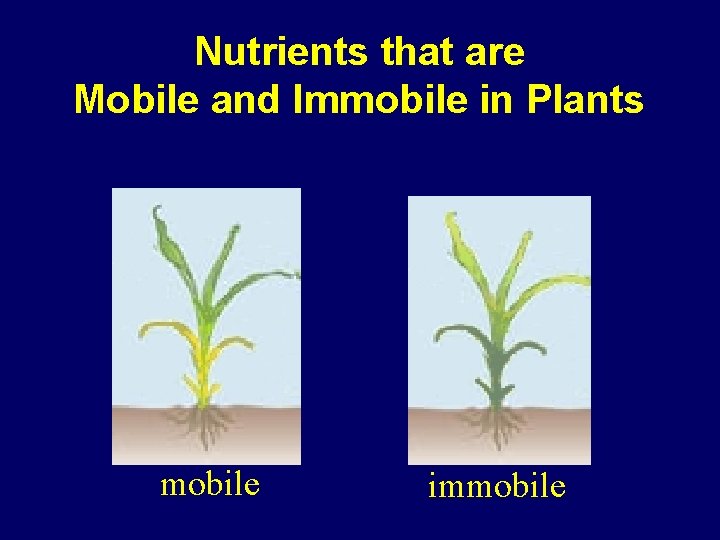
Nutrients that are Mobile and Immobile in Plants mobile immobile

Nitrogen Deficiency


Waterlogged soil resulted in nitrogen loss by denitrification and leaching.

Waterlogged soils and N deficient corn
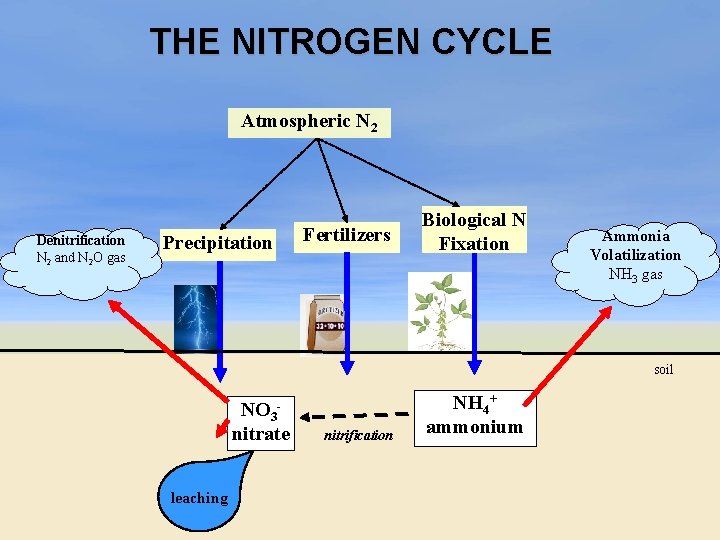
THE NITROGEN CYCLE Atmospheric N 2 Denitrification N 2 and N 2 O gas Precipitation Fertilizers Biological N Fixation Ammonia Volatilization NH 3 gas soil NO 3 nitrate leaching nitrification NH 4+ ammonium

Nitrogen Cycle ü Main reservoir of nitrogen is atmospheric air (78% of air is N 2) ü As N moves through the Nitrogen Cycle, an atom of N may appear in many different chemical forms. (N 2 , NH 4+, NO 3 -) ü N cycle has a gas phase and a mineral phase.

Nitrogen “Fixed” By: 1. Biological Organisms • EX: Rhizobium bacteria / legumes 2. Lightning • Lightning strike “fixes” some nitrogen which comes down in rain. • adds approx. 1 -20 lb per acre annually 3. Nitrogen fertilizers • N containing synthetic fertilizers • bypasses "natural" N fixation
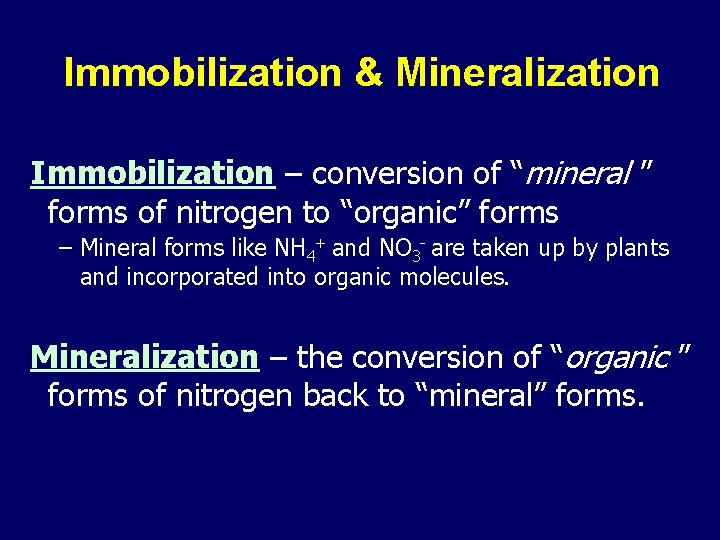
Immobilization & Mineralization Immobilization – conversion of “mineral ” forms of nitrogen to “organic” forms − Mineral forms like NH 4+ and NO 3 - are taken up by plants and incorporated into organic molecules. Mineralization – the conversion of “organic ” forms of nitrogen back to “mineral” forms.

Nitrogen & Organic Matter ü Plants utilize N as ammonium (NH 4+ ) or nitrate (NO 3 -) ü More than 95% of the total nitrogen in the soil is immobilized in OM and released slowly. ü Soil with 1% OM contains about 2, 000 lbs N; but only about 1% mineralizes annually and becomes available for plants (~20 lbs). ü OM decomposition and release of N proceeds most quickly in warm, well-aerated, moist soils
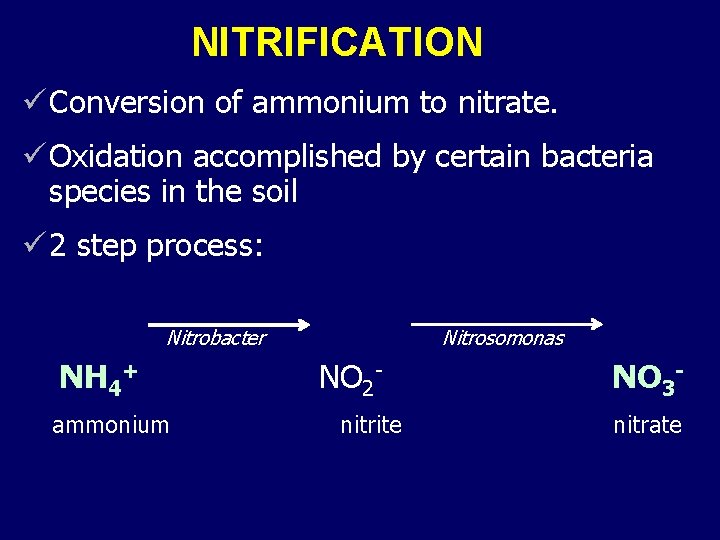
NITRIFICATION ü Conversion of ammonium to nitrate. ü Oxidation accomplished by certain bacteria species in the soil ü 2 step process: Nitrobacter NH 4+ ammonium Nitrosomonas NO 2 nitrite NO 3 nitrate
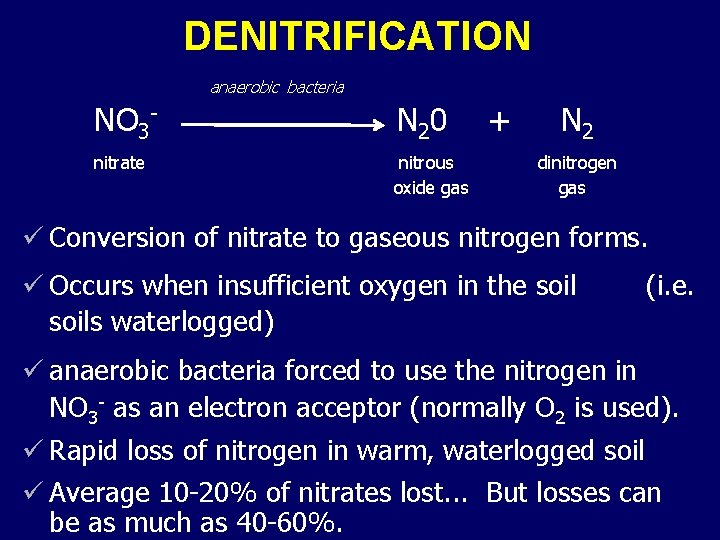
DENITRIFICATION anaerobic bacteria NO 3 - N 20 nitrate nitrous oxide gas + N 2 dinitrogen gas ü Conversion of nitrate to gaseous nitrogen forms. ü Occurs when insufficient oxygen in the soils waterlogged) (i. e. ü anaerobic bacteria forced to use the nitrogen in NO 3 - as an electron acceptor (normally O 2 is used). ü Rapid loss of nitrogen in warm, waterlogged soil ü Average 10 -20% of nitrates lost. . . But losses can be as much as 40 -60%.

Waterlogged soil resulted in nitrogen loss by denitrification and leaching.
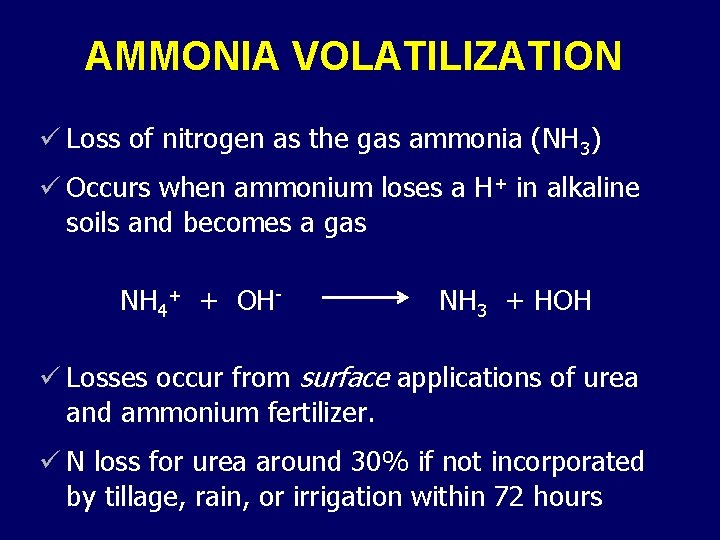
AMMONIA VOLATILIZATION ü Loss of nitrogen as the gas ammonia (NH 3) ü Occurs when ammonium loses a H+ in alkaline soils and becomes a gas NH 4+ + OH- NH 3 + HOH ü Losses occur from surface applications of urea and ammonium fertilizer. ü N loss for urea around 30% if not incorporated by tillage, rain, or irrigation within 72 hours

Fertilizer Guarantee (Grade) %N - %P 2 O 5 - %K 2 O 1. % Total Nitrogen 2. % Available Phosphorus, calculated as P 2 O 5 3. % Water-soluble Potassium, calculated as K 2 O -- Historically, P and K reported as the oxide form. -- Resistance to change because if reported as elemental form the grade would be lower Example: Triple super phosphate 0 -45 -0 is only 0 -19 -0 if reported as %P rather than % P 2 O 5.

The Most Common Nitrogen Containing Fertilizers • • Anhydrous ammonia 82 -0 -0 Urea 46 -0 -0 Ammonium nitrate 34 -0 -0 Ammonium sulfate 21 -0 -0 -24 S UAN 28 -0 -0 or 32 -0 -0 (liquid) Ammonium polyphosphate 10 -34 -0 (liquid) MAP 11 -52 -0 DAP 18 -46 -0

Manage Nitrogen according to Yield Goal ü Nitrogen requirement changes with yield: 30 bushel wheat = 60 lbs N/acre 60 bushel wheat = 125 lbs N/acre 100 bushel wheat = 240 lbs N/acre ü Soil test to determine what you currently have… then add the difference to insure adequate N for your yield goal
Manage Nitrogen according to Yield Goal General Rules-of-Thumb for Wheat 2 lb N per bushel of wheat grain 60 lb N per ton of wheat forage (hay). - 15 lb N/stocker per month grazing. - 30 lb N/acre per 100 lbs beef produced

SULFUR Importance: ü Found in 3 amino acids: Methionine, Cysteine, Cystine ü Needed for making chlorophyll ü Found in vitamins and oils ü Important in plant metabolism
SULFUR ü Healthy plant foliage generally contains 1/10 th as much sulfur as nitrogen. ü 5 to 15 lb/acre usually adequate ü Sulfate ion (SO 42 -) is form used by plants ü SO 42 - is an anion and easily leached

Natural Sources of Sulfur Organic matter • 70 -90% of soil sulfur is in OM Soil minerals • arid soils ─ gypsum Ca. SO 4 • iron pyrite “fools gold” Fe. S 2

Natural Sources of Sulfur Atmospheric sulfur • Air pollutant from burning coal and oil. • Pollution can supply ½ of plants sulfur requirements. • 2 -8 lb/A sulfur added to soil annually from rain in Oklahoma… more in E • Irrigation water can also be high in sulfate • Irrigation water containing 1 ppm sulfate adds 2. 7 lbs S per acre for each acre-foot of irrigation

Sulfur Deficiency Symptoms: ü Chlorosis in NEW leaves (immobile in plant) ü Growth reduction, but not as dramatic as with N. ü Corn, sorghum, cotton high demand for S
Sulfur Deficiency ü Much of the soil’s sulfur has been used up by high yielding crops over the last 50 years and has not been replaced. ü Sulfur content of today’s preferred fertilizers are low. ü Atmospheric sulfur dioxide pollution has diminished. ü Sandy and low OM soils most prone to S deficiencies
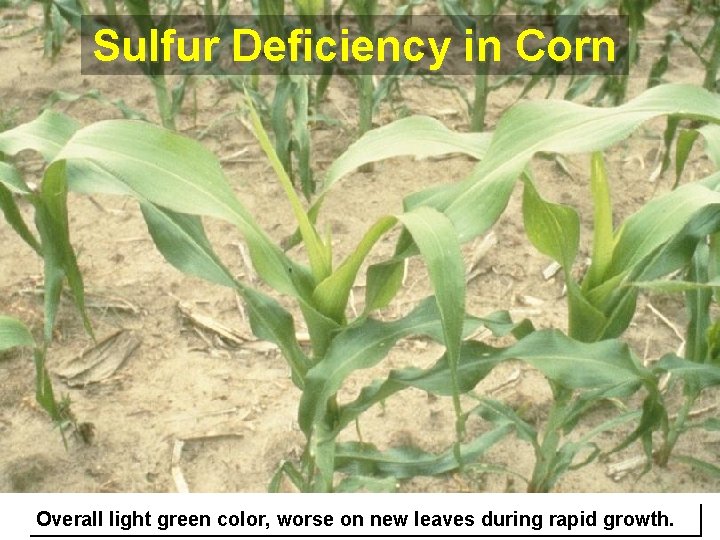
Sulfur Deficiency in Corn Overall light green color, worse on new leaves during rapid growth.

Sulfur Deficiency in Wheat Overall light green color, worse on new leaves during rapid growth.
Similarities of N & S 1. Both held largely in OM 2. Both released as inorganic ions (SO 42 -, NH 4+, NO 3 -); release accomplished by soil microorganisms 3. Anaerobic soil organisms change these elements into gaseous forms. 4. Each have a form that is easily leached (NO 3 - and SO 42 -)

Common Sulfur Fertilizers Material %S Elemental sulfur 90 Ammonium sulfate 24 Ammonium thiosulfate 26 Ordinary superphosphate 12 Potassium sulfate 17 Gypsum 17 Sulfate of potash, magnesia 22
- Slides: 38