NITROGEN AND ITS COMPOUNDS OXIDES OF NITROGEN N



















- Slides: 25

NITROGEN AND ITS COMPOUNDS OXIDES OF NITROGEN: N 2 O, NO 2 FORM: 3 SUBJECT: Chemistry TOPIC: NITROGEN AND ITS COMPOUNDS WEBQUEST SUBTOPIC: OXIDES OF NITROGEN: N 2 O, NO, NO 2

The introduction Introduction There are three oxides of nitrogen: nitrogen (I)oxide, nitrogen (II)oxide and nitrogen (IV)ox The fumes we see from car exhaust engines co nitrogen (IV) oxide. The oxides can be prepared in the laboratory. Nitrogen (II)oxide can be oxidised into nitroge (IV)oxide by atmospheric oxygen.

Task T he task You will be divided into 3 groups named as: Group 1 N 2 O, Group 2 NO, Group 3 NO 2. In your groups you will research on: How the oxide in your group is prepared in the lab. Physical properties (colour, smell, density, nature , boiling point, melting point, collection method). Chemical properties (reaction with oxygen, solubility in water, reaction with hot elements) Uses of the oxides

General N 2 O: https: //www. pinterest. com/pin/3844948869 09210493/ https: //www. youtube. com/watch? v=6 GY 0 V ADh. F 4 k NO: https: //www. pinterest. com/pin/3844948869 09210490/ NO 2: https: //en. wikipedia. org/wiki/Nitrogen_dioxi de https: //science. wonderhowto. com/howto/make-nitrogen-dioxide-lab-301858/ https: //www. pinterest. com/pin/38 4494886909210493/ https: //www. youtube. com/watch?

Objectives: By the end of the lesson the learner should be able to: 1. Name and write the chemical formulae of the three oxides of nitrogen. 2. Explain the laboratory preparation of the three oxides of nitrogen. 3. State and explain both physical and chemical properties of the oxides. 4. Clearly distinguish the oxides based on the properties. 5. State some uses of the oxides.

Ms Word & Ms Power. Point Desktops Internet Laptops Non-ICT Teacher’s notes KLB chemistry book 3 Principles of chemistry bk 3 Apparatus and chemicals in the laboratory

• From the above web linkages extract and compile information into Slides. 1. Explain how the oxides of nitrogen are prepared in the laboratory 2. Explain the collection method of the oxides. 3. Explain both physical and chemical properties of the oxides. 4. Clearly distinguish the oxides based on the properties. 5. State the confirmatory tests of NO and N 2 O. 6. Write some uses of the oxides.

The guidance How are the oxides prepared in the lab? How are the oxides collected? What are the physical and chemical properties of the oxides? How can the oxides be distinguished? What are the confirmatory tests of NO and N 2 O? What are some of the uses of the oxides?

• Summary – the student webquest project • It will involve three stages. • Exposition and launch of the project through webquest slides – 1 lesson - Teacher and Students. • 2. Conduction of project work primarily by students with assistance from Teacher, Computer and Science technicians. - 1 lesson • Compilation of presentation slides and materials, Presentation, evaluation, reflection and ranking of group work – 1 lesson

• Teacher sets the pace and modalities on how groups will present their work and questioning from class members • • The webquest project – student presents • Student project team presentations to class; school community as they seek to answer the following questions • 1. Name the reagents used to prepare the oxides in the laboratory • 2. Name the method used to collect the gases(oxides) and give a reason. • 3. Explain the physical and chemical properties of the oxides. • 4. State the confirmatory tests of NO and N 2 O. • 5. State some of the uses of the oxides.

• Open questions – student defense

• Reflection on the project – pivotal questions • Teacher clarifies or expounds on information and concepts from the groups. • Teacher answers students questions and gives concluding remarks

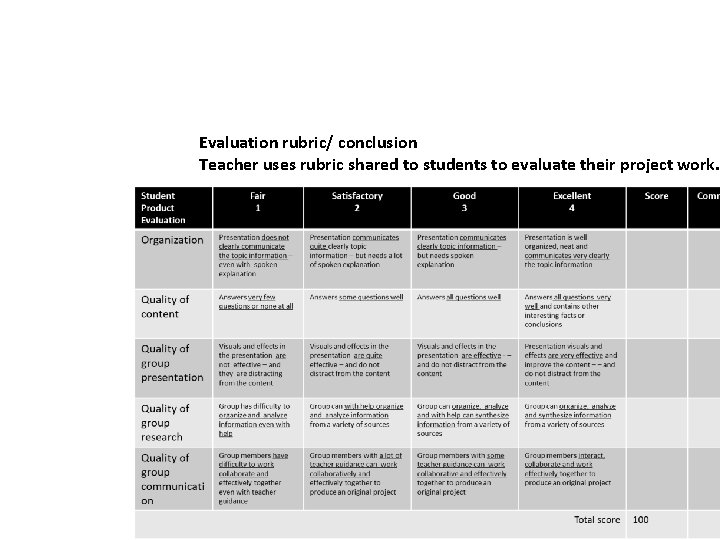
Evaluation rubric/ conclusion Teacher uses rubric shared to students to evaluate their project work.




Resources – Non-ICT

Process

Guidance

Conclusion - Project Presentations

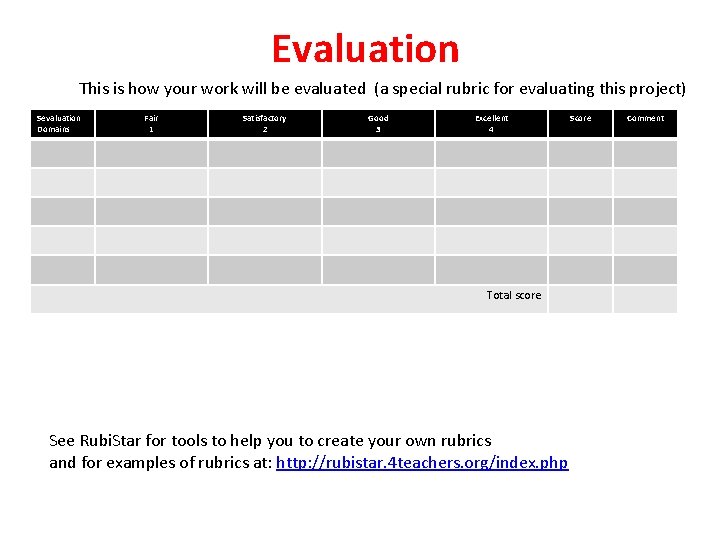
Evaluation This is how your work will be evaluated (a special rubric for evaluating this project) Sevaluation Domains Fair 1 Satisfactory 2 Good 3 Excellent 4 Score Total score See Rubi. Star for tools to help you to create your own rubrics and for examples of rubrics at: http: //rubistar. 4 teachers. org/index. php Comment

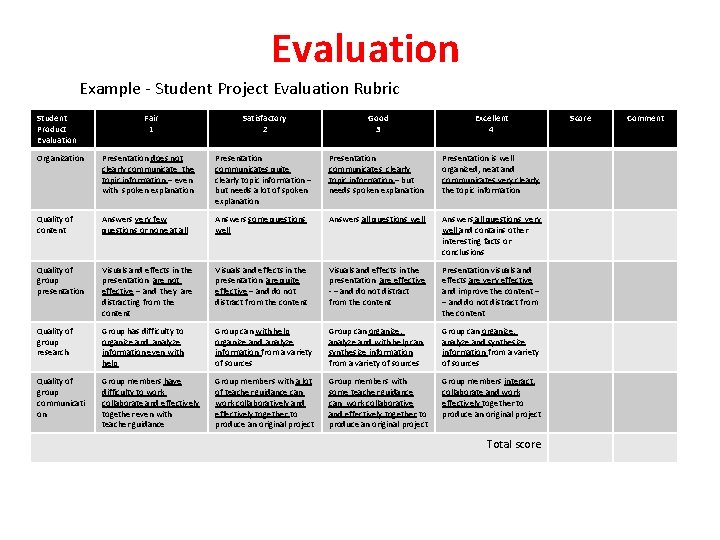
Evaluation Example - Student Project Evaluation Rubric Student Product Evaluation Fair 1 Satisfactory 2 Good 3 Excellent 4 Organization Presentation does not clearly communicate the topic information – even with spoken explanation Presentation communicates quite clearly topic information – but needs a lot of spoken explanation Presentation communicates clearly topic information – but needs spoken explanation Presentation is well organized, neat and communicates very clearly the topic information Quality of content Answers very few questions or none at all Answers some questions well Answers all questions very well and contains other interesting facts or conclusions Quality of group presentation Visuals and effects in the presentation are not effective – and they are distracting from the content Visuals and effects in the presentation are quite effective – and do not distract from the content Visuals and effects in the presentation are effective - – and do not distract from the content Presentation visuals and effects are very effective and improve the content – – and do not distract from the content Quality of group research Group has difficulty to organize and analyze information even with help Group can with help organize and analyze information from a variety of sources Group can organize, analyze and with help can synthesize information from a variety of sources Group can organize, analyze and synthesize information from a variety of sources Quality of group communicati on Group members have difficulty to work collaborate and effectively together even with teacher guidance Group members with a lot of teacher guidance can work collaboratively and effectively together to produce an original project Group members with some teacher guidance can work collaborative and effectively together to produce an original project Group members interact, collaborate and work effectively together to produce an original project Total score Score Comment

Conclusion- Reflection

Examples of Webquests in Science, Technology, English, Mathematics and other subjects QUESTGARDEN

Examples of Webquests Quest. Garden • This is an online website for creating and finding examples of webquests at: http: //questgarden. com/ • Follow the link ‘Search for examples’ for finding hundres of examples of STEM webquests at: http: //questgarden. com/author/examplestop. php • Use the ‘Curriculum x Grade Level Matrix tool’ to find examples of weqbuests in: – 1) your subject area – 2) your grade level (6 -9 grade = middle high school; 9 -12 grade = senior high school)