NITRILE Blessie Mae R Encila IVB S Chemistry

NITRILE Blessie Mae R. Encila IV-B. S Chemistry for Teachers Advance Organic Chemistry

01 WINTER Template Nitrile History Nomenclature

• A nitrile is any organic compound that has a -C≡N functional group. • The prefix cyano- is used interchangeably with the term nitrile in industrial literature. • Nitriles are found in many useful compounds, including Nitrile – methyl cyanoacrylate • used in super glue – nitrile butadiene rubber • a nitrile-containing polymer used in latex-free laboratory and medical gloves.

Nitrile • Organic compounds containing multiple nitrile groups are known as cyanocarbons. • Inorganic compounds containing the -C≡N group are not called nitriles, but cyanides instead. • Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic.

Nitrile • C. W. Scheele in 1782 • J. L. Gay. Lussac in 1811 • Friedrich Wöhler and Justus von Liebig History -He synthesized the first compound of the homolog row of nitriles, which is the nitrile of formic acid, and hydrogen cyanide. -He was able to prepare the very toxic and volatile pure acid. -They prepared the nitrile of benzoic acids, but due to minimal yield of the synthesis neither physical nor chemical properties were determined nor a structure suggested.

Nitrile History • Théophile. Jules Pelouze in 1834 -He synthesized propionitrile suggesting it to be an ether of propionic alcohol and hydrocyanic acid. • Hermann Fehling in 1844 -The synthesis of benzonitrile, by heating ammonium benzoate, was the first method yielding enough of the substance for chemical research. He determined the structure by comparing it to the already known synthesis of hydrogen cyanide by heating ammonium formate to his results. He coined the name nitrile for the newfound substance, which became the name for the compound group.
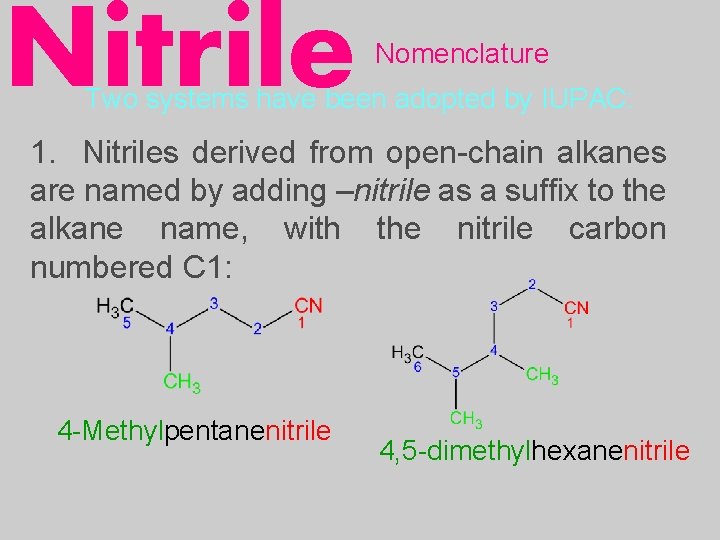
Nitrile Nomenclature Two systems have been adopted by IUPAC: 1. Nitriles derived from open-chain alkanes are named by adding –nitrile as a suffix to the alkane name, with the nitrile carbon numbered C 1: 4 -Methylpentanenitrile 4, 5 -dimethylhexanenitrile
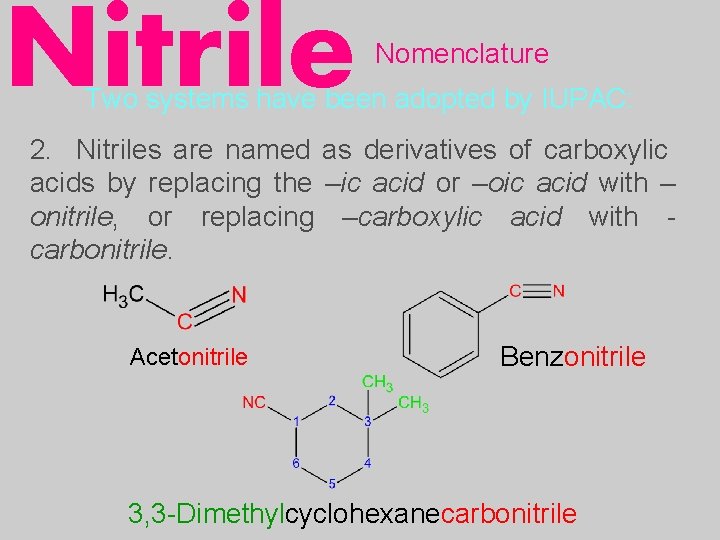
Nitrile Nomenclature Two systems have been adopted by IUPAC: 2. Nitriles are named as derivatives of carboxylic acids by replacing the –ic acid or –oic acid with – onitrile, or replacing –carboxylic acid with carbonitrile. Acetonitrile Benzonitrile 3, 3 -Dimethylcyclohexanecarbonitrile

02 Nitrile General Reactions of Nitrile
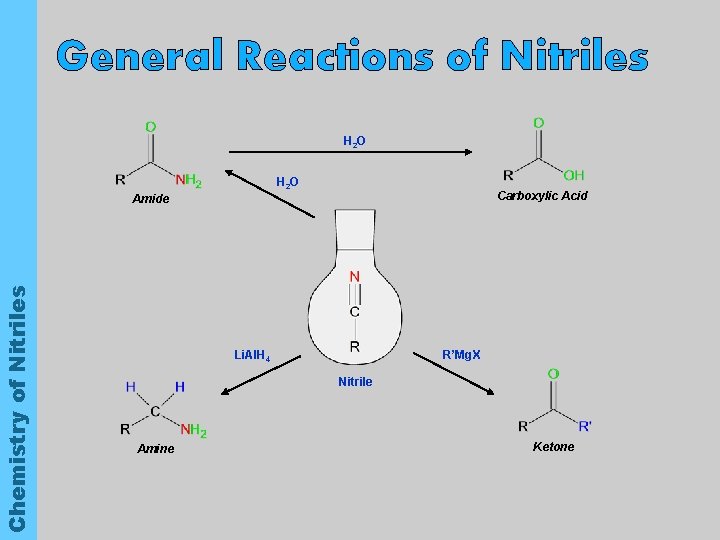
General Reactions of Nitriles H 2 O Carboxylic Acid Chemistry of Nitriles Amide Li. Al. H 4 R’Mg. X Nitrile Amine Ketone
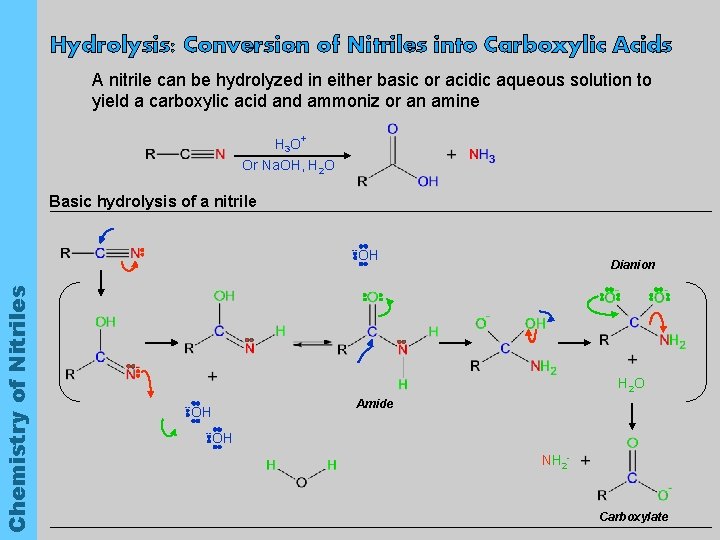
Hydrolysis: Conversion of Nitriles into Carboxylic Acids A nitrile can be hydrolyzed in either basic or acidic aqueous solution to yield a carboxylic acid and ammoniz or an amine H 3 O+ Or Na. OH, H 2 O Basic hydrolysis of a nitrile Chemistry of Nitriles -- OH Dianion H 2 O -- Amide OH -- OH NH 2 - Carboxylate

Reduction: Conversion of Nitriles into Amines Chemistry of Nitriles A nitrile can be reduced with Li. Al. H 4 to give a primary amine Li. Al. H 4 ether Nitrile H 2 O Li. Al. H 4 ether Imine anion Dianion Amine
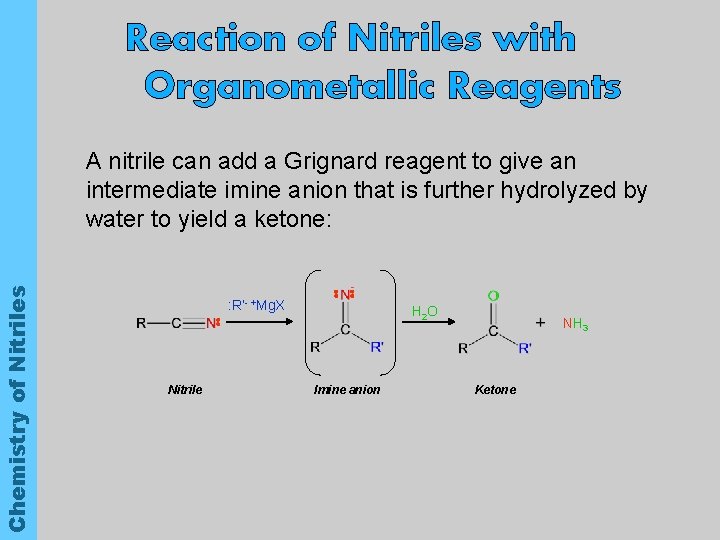
Reaction of Nitriles with Organometallic Reagents Chemistry of Nitriles A nitrile can add a Grignard reagent to give an intermediate imine anion that is further hydrolyzed by water to yield a ketone: : R’- +Mg. X Nitrile H 2 O Imine anion NH 3 Ketone

03 Nitrile Application

• Over 30 nitrile-containing pharmaceuticals are currently marketed for a diverse variety of medicinal indications with more than 20 additional nitrile-containing leads in clinical development. Nitrile
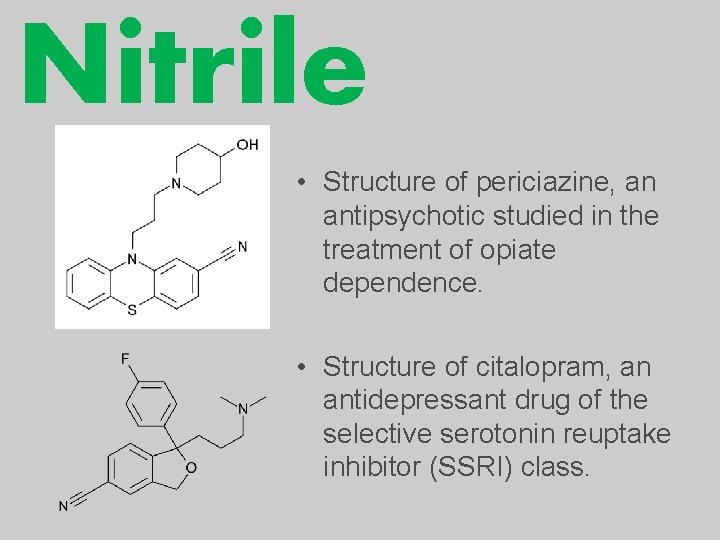
Nitrile • Structure of periciazine, an antipsychotic studied in the treatment of opiate dependence. • Structure of citalopram, an antidepressant drug of the selective serotonin reuptake inhibitor (SSRI) class.
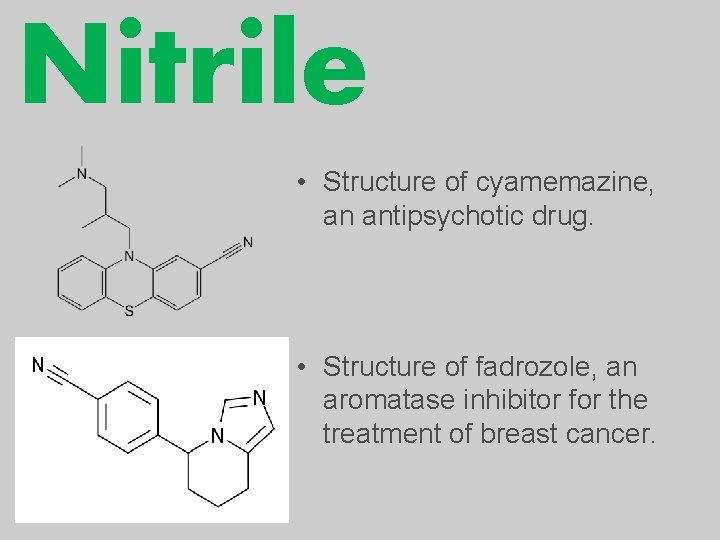
Nitrile • Structure of cyamemazine, an antipsychotic drug. • Structure of fadrozole, an aromatase inhibitor for the treatment of breast cancer.
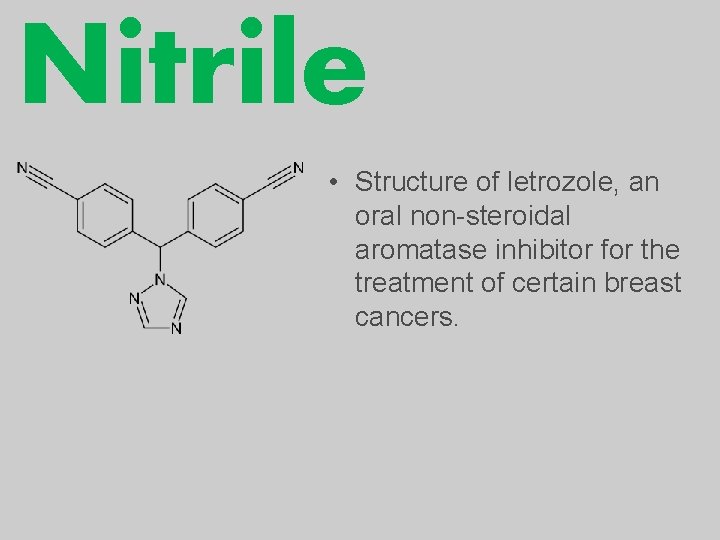
Nitrile • Structure of letrozole, an oral non-steroidal aromatase inhibitor for the treatment of certain breast cancers.

04 Nitrile Journal

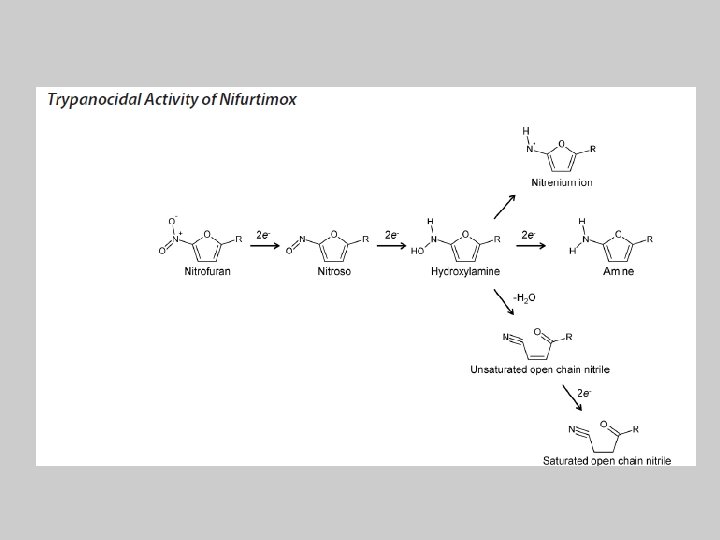
Nifurtimox Activation by Trypanosomal Type I Nitroreductases Generates Cytotoxic Nitrile Metabolites Belinda S. Hall, Christopher Bot, and Shane R. Wilkinson From the School of Biological and Chemical Sciences, Queen Mary University of London, United Kingdom

• The aim of this work was to determine the role of NTRs (trypanosomal type I nitroreductase) in nifurtimox action.

05 Thank You!
- Slides: 23