NIST Real Fuels Chemical Kinetic Combustion Model Database
NIST Real Fuels Chemical Kinetic Combustion Model Database D. R. Burgess, Jr. , T. C. Allison, J. A. Manion, and W. Tsang Physical and Chemical Properties Division National Institute of Standards and Technology Real Fuels • Transportation, Aviation fuels – gasoline, diesel, jet • Mixtures of n-paraffins, iso-paraffins, olefins, naphthenes, aromatics • Individual component composition: Gas(C 4 -C 11), Diesel/Jet (C 9 -C 15) Model Complexity • Methane/O 2 : • Heptane/O 2: several dozen species 100’s of species 100 some reactions 1000’s of reactions Problem • for small molecules – keeping track of data is not too difficult • for large, complex systems - efficient data management is problematic

Information Overload means • Time Consuming “Bookkeeping Chores” just don’t get done • Compromised Quality in results • Data Management is a “rate-limiting step” in the development of robust, validated models

State of the Art for Combustion Modeling High Performance Tools and Advances • Advanced Comput. Fluid Dynamics (CFD) • Quantum Chemical Methods • Master Equation Analysis • Automated Mechanism Generation • Mechanism Reduction Tools • Understanding details of PAH formation • etc.

State of the Art for Combustion Modeling High Performance Tools and Advances • Advanced Comput. Fluid Dynamics (CFD) • Quantum Chemical Methods • Master Equation Analysis • Automated Mechanism Generation • Mechanism Reduction Tools • Understanding details of PAH formation • etc. Archaic Data Management • 19 th century (computer-wise) paradigms • Flat text files • Formats traceable to ANSI Fortran (1977) • Minimal error checking procedures • Minimal electronic “pedigree” documentation • Ad hoc symbolic species/reactions notation • etc.
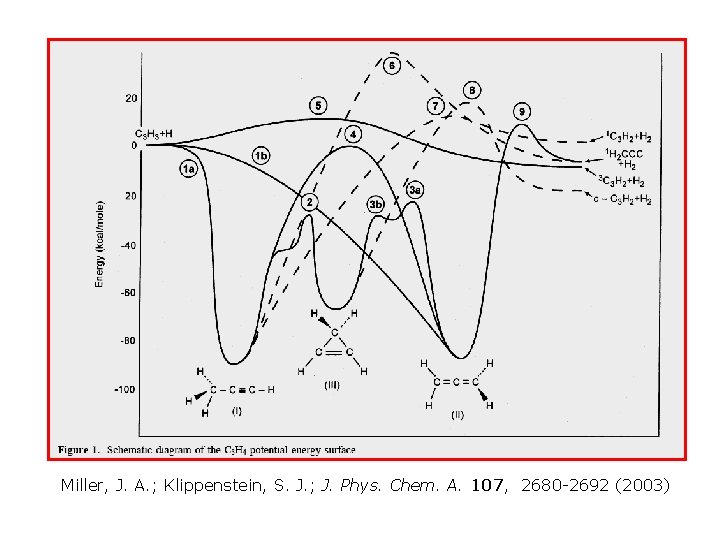
Miller, J. A. ; Klippenstein, S. J. ; J. Phys. Chem. A. 107, 2680 -2692 (2003)

C 2 H 6 L 8/88 C 2 H 6 00 00 G 200. 000 3500. 000 1000. 000 1. 07188150 E+00 2. 16852677 E-02 -1. 00256067 E-05 2. 21412001 E-09 -1. 90002890 E-13 -1. 14263932 E+04 1. 51156107 E+01 4. 29142492 E+00 -5. 50154270 E-03 5. 99438288 E-05 -7. 08466285 E-08 2. 68685771 E-11 -1. 15222055 E+04 2. 66682316 E+00 1. 18915940 E+04 C 6 H 8 H 6 W/94 C 6 H 8 0 0 G 300. 000 3000. 000 0. 28481979 E+02 -0. 15702948 E-01 0. 26771697 E-04 -0. 11780109 E-07 0. 16573427 E-11 0. 93346445 E+04 -0. 12500226 E+03 0. 15850439 E+01 0. 40215142 E-01 0. 78439543 E-05 -0. 38761325 E-07 0. 18545207 E-10 0. 17949613 E+05 0. 19112625 E+02 i-C 4 H 5 H 6 W/94 C 4 H 5 0 0 G 300. 000 3000. 000 0. 10229092 E+02 0. 94850138 E-02 -0. 90406445 E-07 -0. 12596100 E-08 0. 24781468 E-12 0. 34642812 E+05 -0. 28564529 E+02 -0. 19932900 E-01 0. 38005672 E-01 -0. 27559450 E-04 0. 77835551 E-08 0. 40209383 E-12 0. 37496223 E+05 0. 24394241 E+02 HCCHCCH 82489 C 4 H 3 g 0300. 00 4000. 00 1000. 00 0. 01075274 e+03 0. 05381153 e-01 -0. 05549638 e-05 -0. 03052266 e-08 0. 05761740 e-12 0. 06121419 e+06 -0. 02973025 e+03 0. 04153882 e+02 0. 01726287 e+00 -0. 02389374 e-05 -0. 01018700 e-06 0. 04340505 e-10 0. 06338071 e+06 0. 06036507 e+02 c. ccho 11/12/97 c 3 h 5 o 1 0 g 300. 000 5000. 000 1371. 000 9. 97669730 e+00 1. 15899378 e-02 -3. 93593247 e-06 6. 08601507 e-10 -3. 52246874 e-14 -3. 71966345 e+03 -2. 42959564 e+01 2. 95202753 e+00 2. 48297051 e-02 -1. 23487153 e-05 2. 49449869 e-09 -8. 44820686 e-14 -9. 23323349 e+02 1. 46236244 e+01 me. Cy 14 pd 3 bozzelli C 6 H 7 0 0 g 300. 000 5000. 000 1352. 000 1. 64509401 e+01 1. 80104599 e-02 -6. 44365088 e-06 1. 03095614 e-09 -6. 10814842 e-14 1. 54366624 e+04 -6. 72601234 e+01 -9. 87511632 e-01 4. 83156342 e-02 -2. 09426592 e-05 6. 71221112 e-10 1. 09340225 e-12 2. 25173499 e+04 3. 00704019 e+01 1 2 3 4 1 2 3 4

NIST Chemical Kinetic Combustion Model Database This unified Web site will provide the combustion community with detailed chemical kinetic models, along with supporting information, used in modeling applications. ü Chemical Kinetic Models used for combustion simulations ü Thermochemical Data for fuel components & reaction intermediates ü Elementary Rate Coefficients for chemical reactions relevant to combustion • • • o o The primary focus of the Web site is to provide a centralized source of information where well-documented, annotated reaction sets can be downloaded or uploaded. Particular emphasis is given towards promoting standards for nomenclature, notation, traceability, and communication in the modeling community. This includes providing systematic classification schemes for molecular species and chemical reactions. NIST not developing models – just centralized source for data Database will NOT warehouse ALL data – will provide links for some information

NIST Chemical Kinetic Combustion Model Database • • Current site is working prototype Main current tasks are collecting and organizing data NIST is seeking input and guidance from the combustion community with regard to its development in order to improve its content and capabilities. http: //kinetics. nist. gov/CKMech http: //kinetics. nist. gov/realfuels
One of a Set of Tools under Development in the Community The intent for this site and database is • To be complementary to other mechanism, modeling, and data analysis tools under development • To target areas not being substantially addressed elsewhere such as standards, nomenclature, notation, traceability, etc. • To collect and organize data to be used eventually in community wide data evaluation process (akin to IUPAC, JPL atmospheric eval’s, but electronic) • To facilitate collaboration among the community with regard to process modeling • To be integrated into developing infrastructure in the chemical sciences
Related Complementary Efforts EVALUATION • • JPL Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies IUPAC Subcommittee for Gas Kinetic Evaluation TOOLS • • Pr. IMe – Process Informatics Model CMCS – Collaboratory for Multi-Scale Chemical Science RIOT – Range Identification and Optimization Tool for Kinetic Modeling (MIT) MECHMOD (Leeds) DATA • • Burcat’s Thermochemical Database Chemkin Thermochemical Database (Sandia/Reaction Design) GRI-Mech Combustion Model SNL High Temperature Thermodynamics Database LLNL Combustion Chemical Kinetic Mechanisms Cal Tech Reaction Mechanism Library compilation NIST Chemistry Webbook NIST Chemical Kinetics Database

NIST Detailed Chemical Kinetic Combustion Model Database Overview • • Chemical terminology, definitions, etc. Links to related effort, etc Models 1) 2) Archival "flat file" listings of detailed chemical kinetic models with brief descriptions Dynamical "relational" datasets of detailed chemical kinetic models with links to species, reaction, and bibliographic information Bibliography • Supporting documentation for models, reactions, and species Species • • Molecular identifiers such as CASNO, chemical names, etc Molecular structures and chemical information such as structural/chemical formulae, SMILES strings, NICh. I identifiers, MDL mol files, chemical classes, etc. Reactions • Tools to search, manipulate, and compare individual reactions or reactions by class for specific detailed kinetic models or among models or build a model based on recommended values
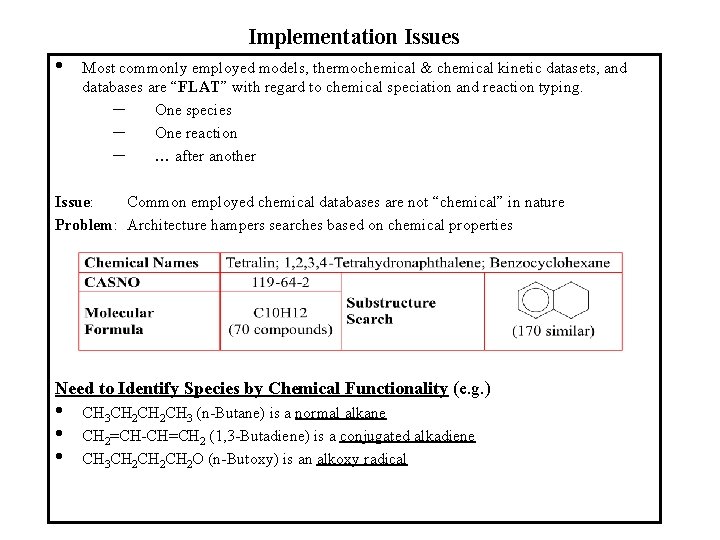
Implementation Issues • Most commonly employed models, thermochemical & chemical kinetic datasets, and databases are “FLAT” with regard to chemical speciation and reaction typing. – One species – One reaction – … after another Issue: Common employed chemical databases are not “chemical” in nature Problem: Architecture hampers searches based on chemical properties Need to Identify Species by Chemical Functionality (e. g. ) • • • CH 3 CH 2 CH 3 (n-Butane) is a normal alkane CH 2=CH-CH=CH 2 (1, 3 -Butadiene) is a conjugated alkadiene CH 3 CH 2 CH 2 O (n-Butoxy) is an alkoxy radical
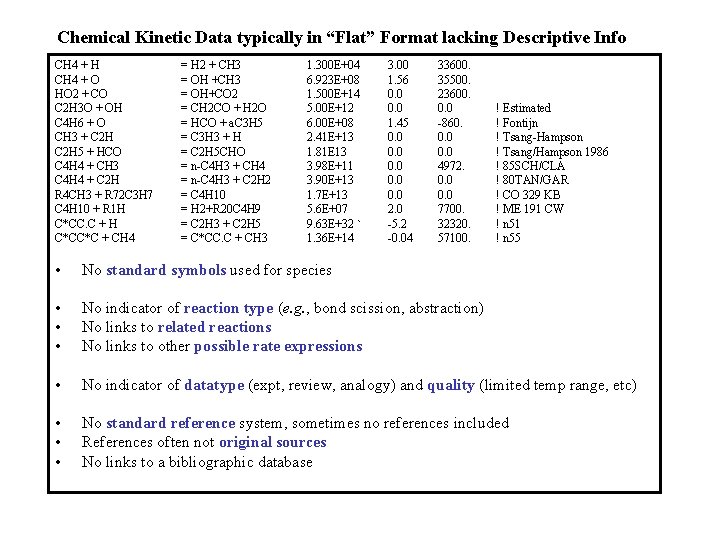
Chemical Kinetic Data typically in “Flat” Format lacking Descriptive Info CH 4 + H CH 4 + O HO 2 + CO C 2 H 3 O + OH C 4 H 6 + O CH 3 + C 2 H 5 + HCO C 4 H 4 + CH 3 C 4 H 4 + C 2 H R 4 CH 3 + R 72 C 3 H 7 C 4 H 10 + R 1 H C*CC. C + H C*CC*C + CH 4 = H 2 + CH 3 = OH +CH 3 = OH+CO 2 = CH 2 CO + H 2 O = HCO + a. C 3 H 5 = C 3 H 3 + H = C 2 H 5 CHO = n-C 4 H 3 + CH 4 = n-C 4 H 3 + C 2 H 2 = C 4 H 10 = H 2+R 20 C 4 H 9 = C 2 H 3 + C 2 H 5 = C*CC. C + CH 3 1. 300 E+04 6. 923 E+08 1. 500 E+14 5. 00 E+12 6. 00 E+08 2. 41 E+13 1. 81 E 13 3. 98 E+11 3. 90 E+13 1. 7 E+13 5. 6 E+07 9. 63 E+32 ` 1. 36 E+14 3. 00 1. 56 0. 0 1. 45 0. 0 0. 0 2. 0 -5. 2 -0. 04 33600. 35500. 23600. 0. 0 -860. 0. 0 4972. 0. 0 7700. 32320. 57100. ! Estimated ! Fontijn ! Tsang-Hampson ! Tsang/Hampson 1986 ! 85 SCH/CLA ! 80 TAN/GAR ! CO 329 KB ! ME 191 CW ! n 51 ! n 55 • No standard symbols used for species • • • No indicator of reaction type (e. g. , bond scission, abstraction) No links to related reactions No links to other possible rate expressions • No indicator of datatype (expt, review, analogy) and quality (limited temp range, etc) • • • No standard reference system, sometimes no references included References often not original sources No links to a bibliographic database
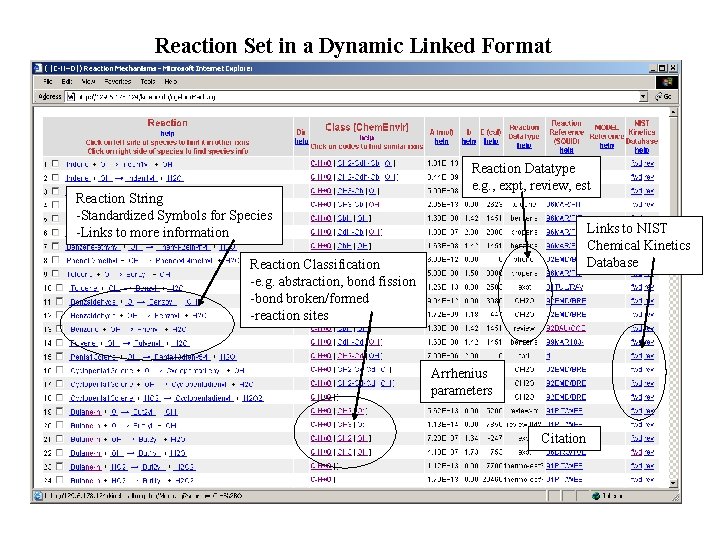
Reaction Set in a Dynamic Linked Format Reaction String -Standardized Symbols for Species -Links to more information Reaction Datatype e. g. , expt, review, est Links to NIST Chemical Kinetics Database Reaction Classification -e. g. abstraction, bond fission -bond broken/formed -reaction sites Arrhenius parameters Citation
http: //kinetics. nist. gov/CKMech
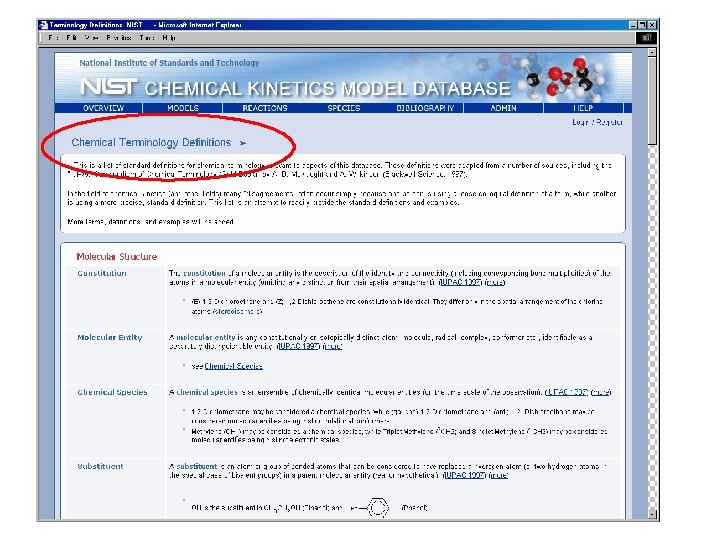
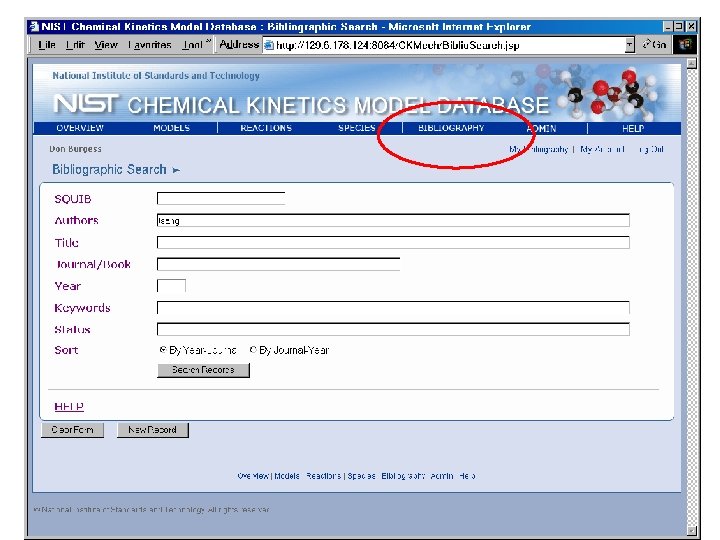
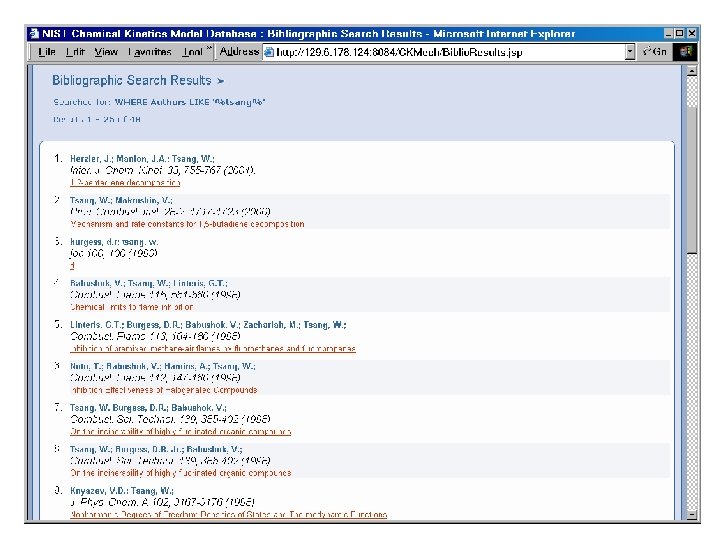
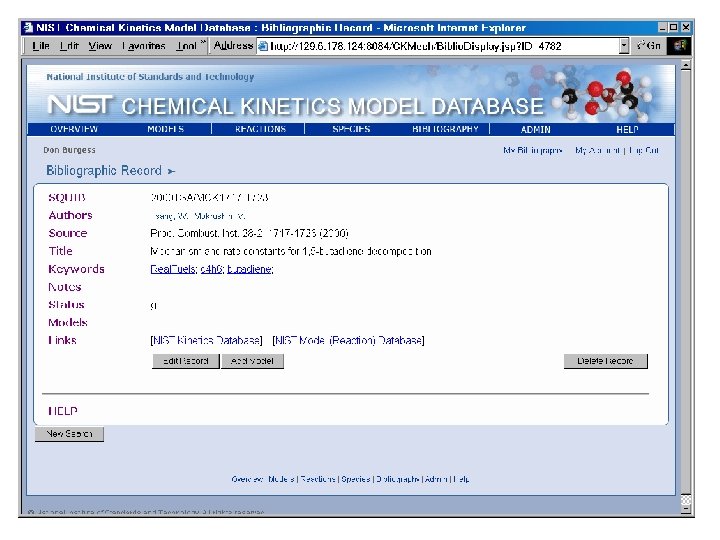
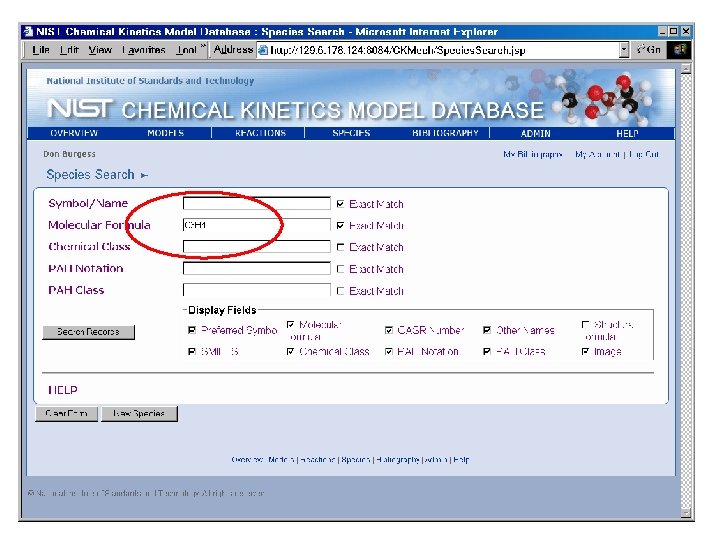
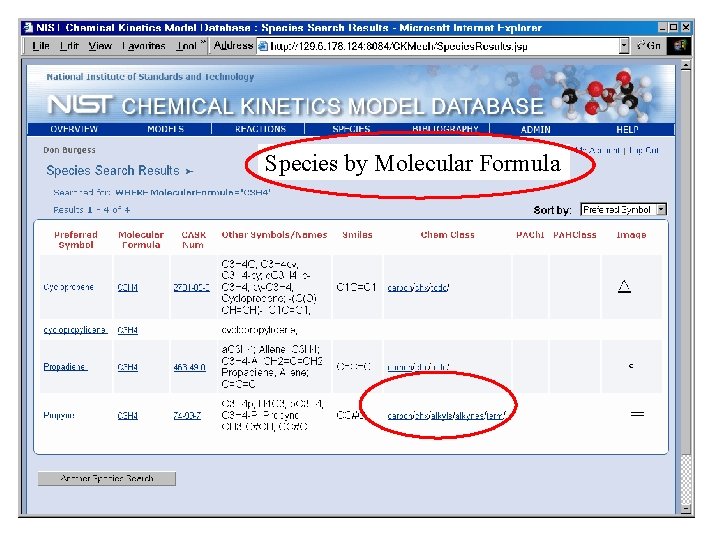
Species by Molecular Formula
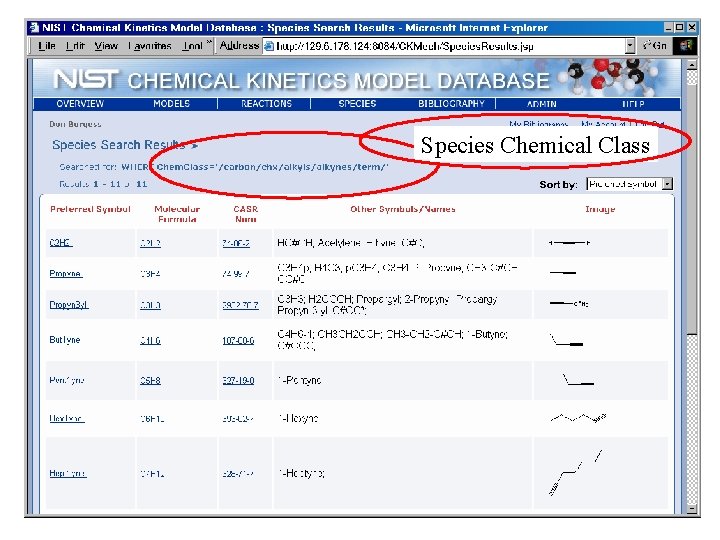
Species Chemical Class
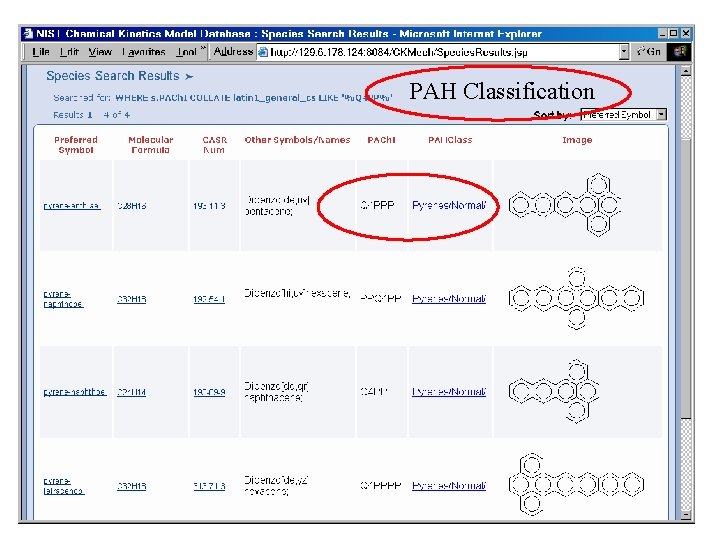
PAH Classification
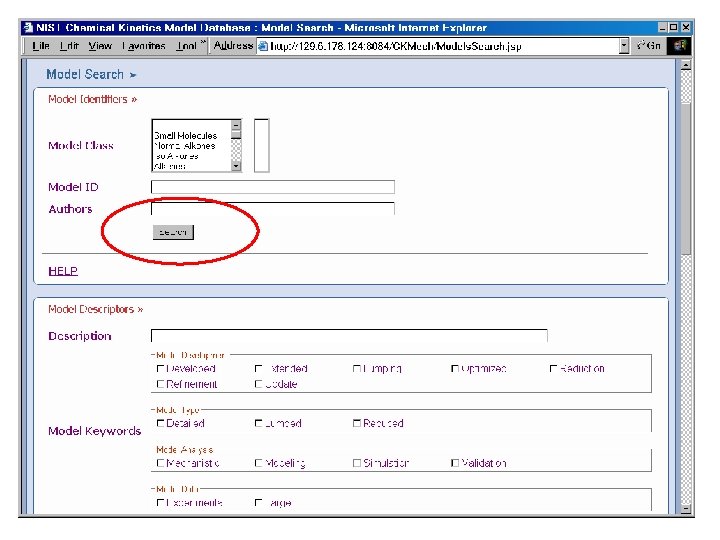
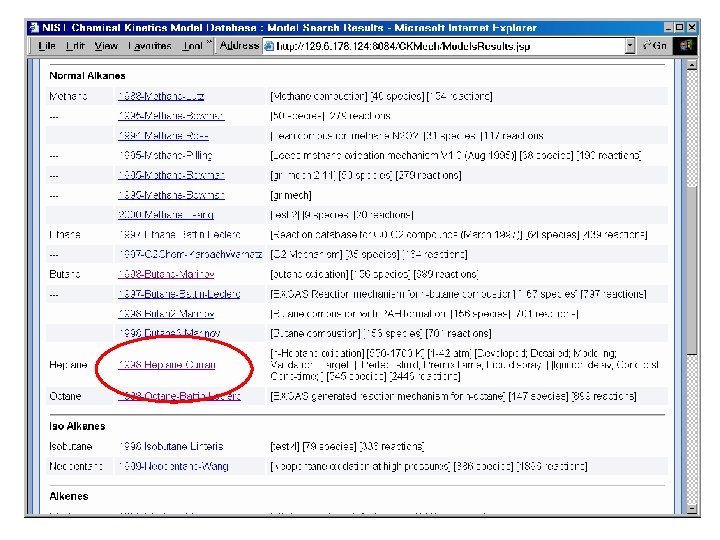
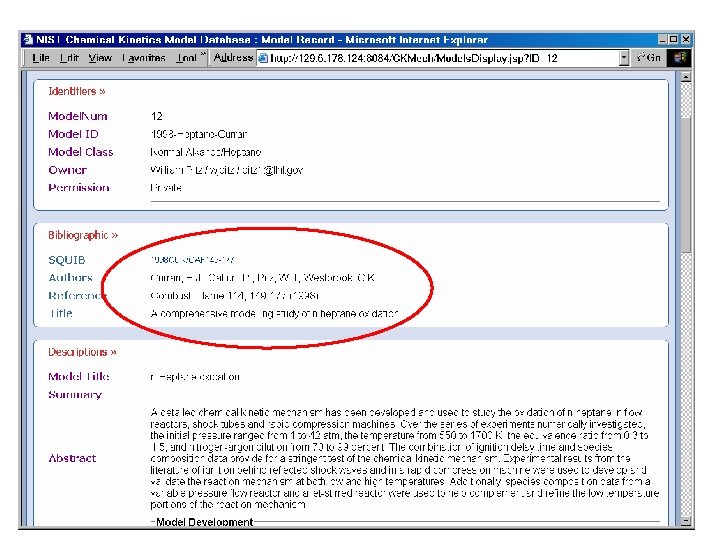
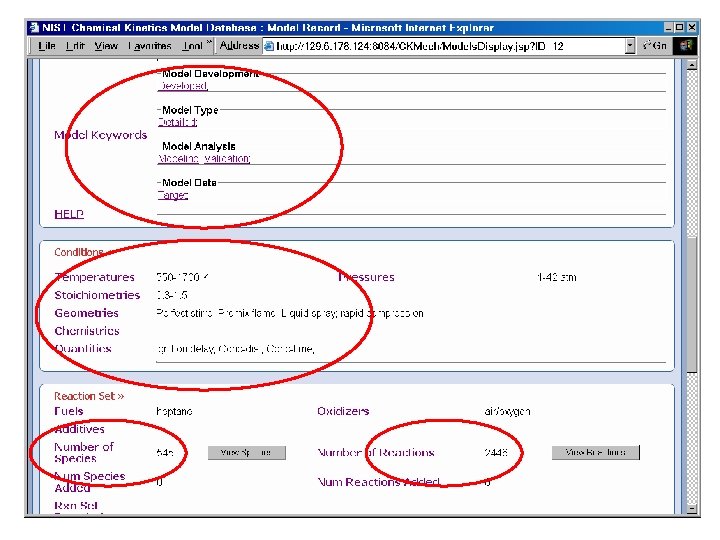
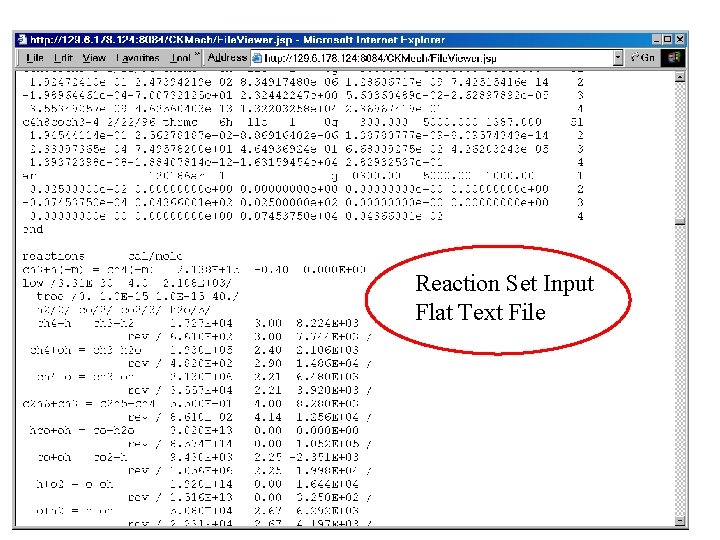
Reaction Set Input Flat Text File
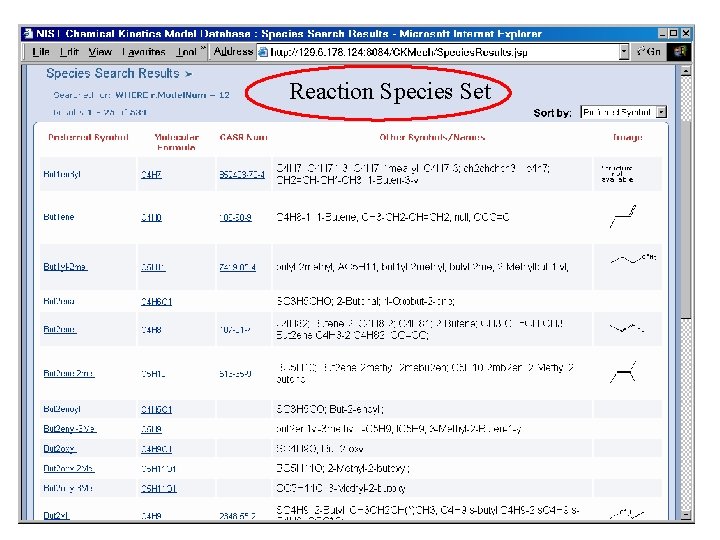
Reaction Species Set
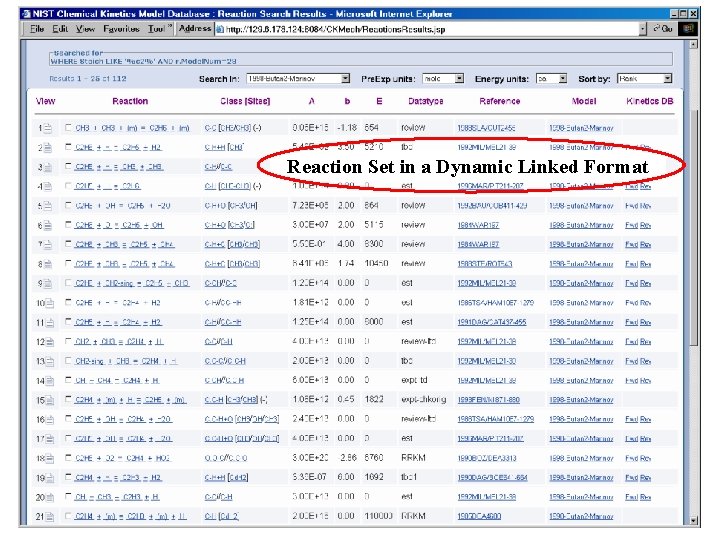
Reaction Set in a Dynamic Linked Format
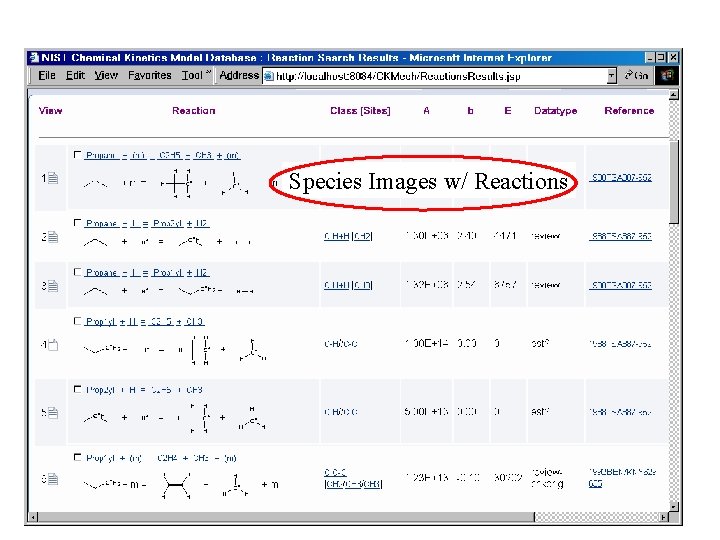
Species Images w/ Reactions
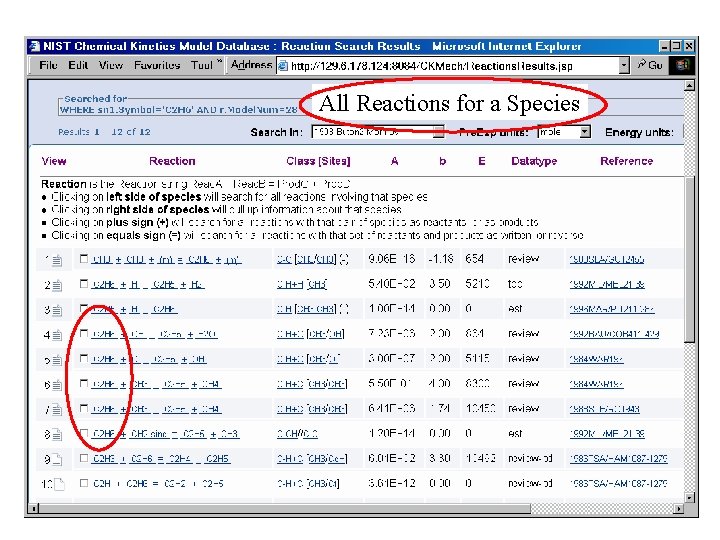
All Reactions for a Species
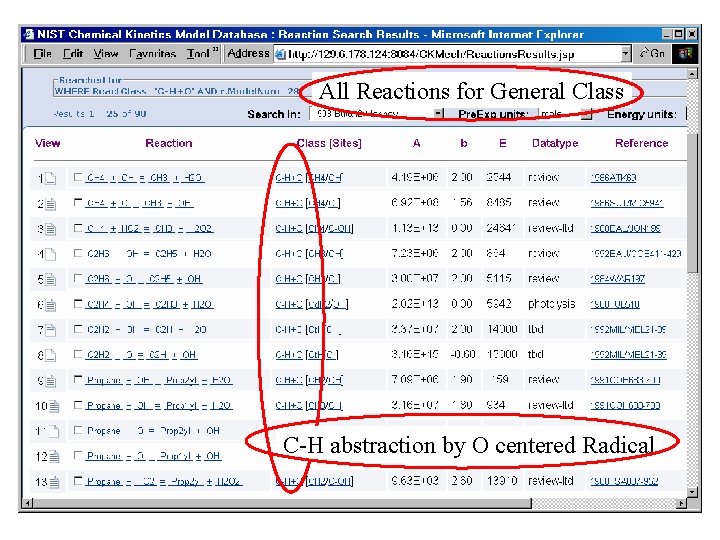
All Reactions for General Class C-H abstraction by O centered Radical
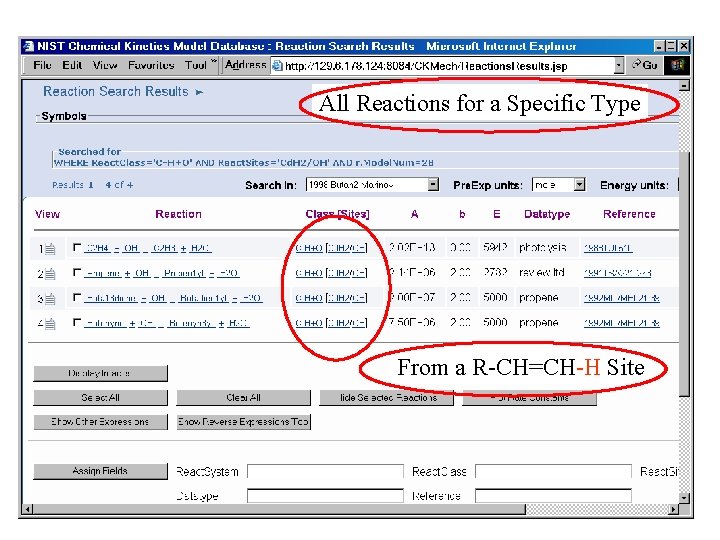
All Reactions for a Specific Type From a R-CH=CH-H Site
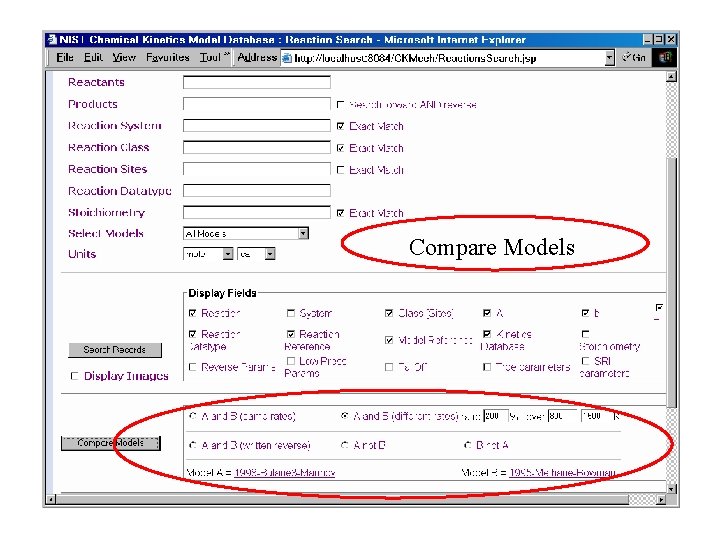
Compare Models
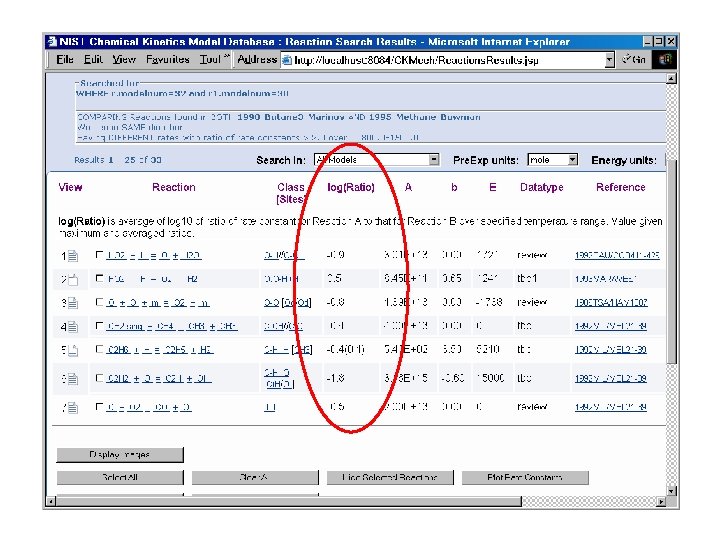
NIST Chemical Kinetic Combustion Model Database This unified Web site will provide the combustion community with detailed chemical kinetic models, along with supporting information, used in modeling applications. ü Chemical Kinetic Models used for combustion simulations ü Thermochemical Data for fuel components & reaction intermediates ü Elementary Rate Coefficients for chemical reactions relevant to combustion • • • o o The primary focus of the Web site is to provide a centralized source of information where well-documented, annotated reaction sets can be downloaded or uploaded. Particular emphasis is given towards promoting standards for nomenclature, notation, traceability, and communication in the modeling community. This includes providing systematic classification schemes for molecular species and chemical reactions. NIST not developing models – just centralized source for data Database will NOT warehouse ALL data – will provide links for some information

NIST Chemical Kinetic Combustion Model Database • • Current site is working prototype Main current tasks are collecting and organizing data NIST is seeking input and guidance from the combustion community with regard to its development in order to improve its content and capabilities. http: //kinetics. nist. gov/CKMech http: //kinetics. nist. gov/realfuels
- Slides: 38