NIOSH Town Hall Meeting CSTD Testing Protocol November

NIOSH Town Hall Meeting CSTD Testing Protocol November 7, 2016 Cinncinnati, Ohio Firouzan ‘Fred’ Massoomi, Pharm. D. , FASHP Pharmacist 5618 Nicholas St. Omaha, NE 68132 massoomi@cox. net Submitted by: Fred Massoomi, Pharm. D. , FASHP

NIOSH • I want to first acknowledge and thank NIOSH as a healthcare provider for devoting their efforts to our safety. • Although, we in this room know the risk of associated with Healthcare’s Dirty Little secret it is still not fully accepted. • Regulatory actions of states and UPS 800 are accepted more freely versus the evidence Submitted by: Fred Massoomi, Pharm. D. , FASHP

CSTD Manufactures • I want to acknowledge and thank all CSTD manufactures for devoting resources to our safety. • It has been a pleasure to watch these devices evolve of the past 16 years • The traditional needle and syringe methodology is dangerous in many ways. Submitted by: Fred Massoomi, Pharm. D. , FASHP

Published Issues with This Methodology Submitted by: Fred Massoomi, Pharm. D. , FASHP

FDA Cleared CSTDs Device Manufacturer FDA Cleared Pha. Seal® Becton, Dickinson and Company; Carmel Pharma, Inc. (original) 1998 Spiros® ICU Medical, Inc. 2005 Texium® with Smart. Site® Becton, Dickinson and Company; Care. Fusion, Inc. (original) 2006 Tevadaptor® (On. Guard®) B. Braun Medical Inc. (US distributor) TEVA Medical, Ltd. (manufacturer) 2006 Chemo. Clave® ICU Medical, Inc. 2008 Equashield® Equashield, LLC; Plastmed, Ltd (original) 2009 Chemo. Lock® ICU Medical, Inc. 2013 Chemo. Safety® Becton, Dickinson and Company; Care. Fusion (original) 2013 Equashield II® Equashield, LLC 2014 Halo® Corvida Medical 2015 Modified from: Massoomi F. The evolution of the CSTD. Pharm Purch & Prod. 2015 Feb; 12(2): S 1 -S 12. Submitted by: Fred Massoomi, Pharm. D. , FASHP
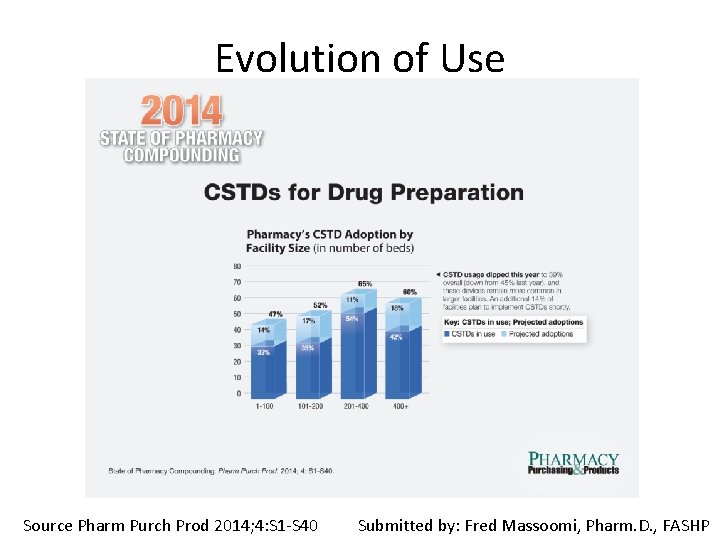
Evolution of Use Source Pharm Purch Prod 2014; 4: S 1 -S 40 Submitted by: Fred Massoomi, Pharm. D. , FASHP
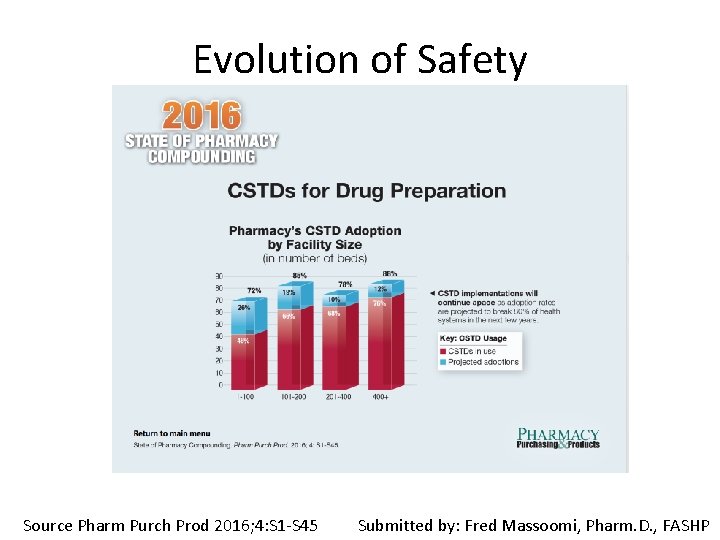
Evolution of Safety Source Pharm Purch Prod 2016; 4: S 1 -S 45 Submitted by: Fred Massoomi, Pharm. D. , FASHP
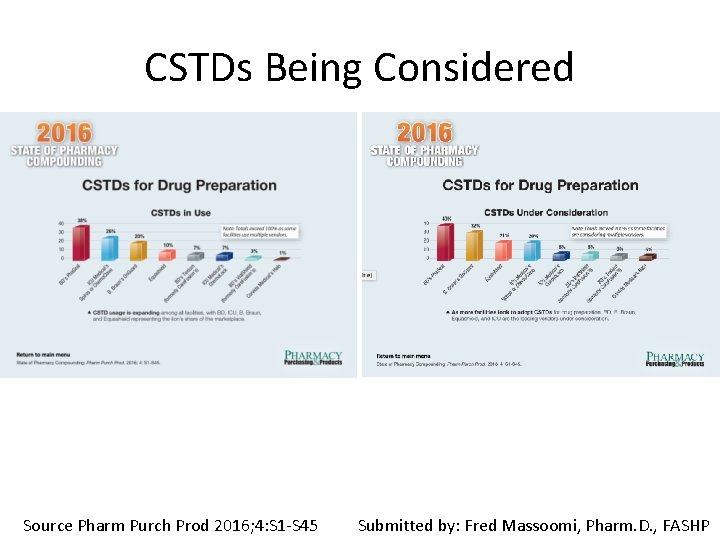
CSTDs Being Considered Source Pharm Purch Prod 2016; 4: S 1 -S 45 Submitted by: Fred Massoomi, Pharm. D. , FASHP

CSTD* Testing Comments • To date, no proposed NIOSH test adequately assesses all FDA cleared CSTDs* in a clinical manner • NIOSH proposed tests are difficult to repeat in clinical settings and are not economical • Many tests developed by independent researchers emulate clinical use and are easily repeatable • A first test to consider is confirmation of dry connections – If it is not dry, it will not contain vapors – Easy to repeat at clinical sites and economical • Markers: p. H variations of drugs and fluorescence *Closed System Transfer Devices Submitted by: Fred Massoomi, Pharm. D. , FASHP
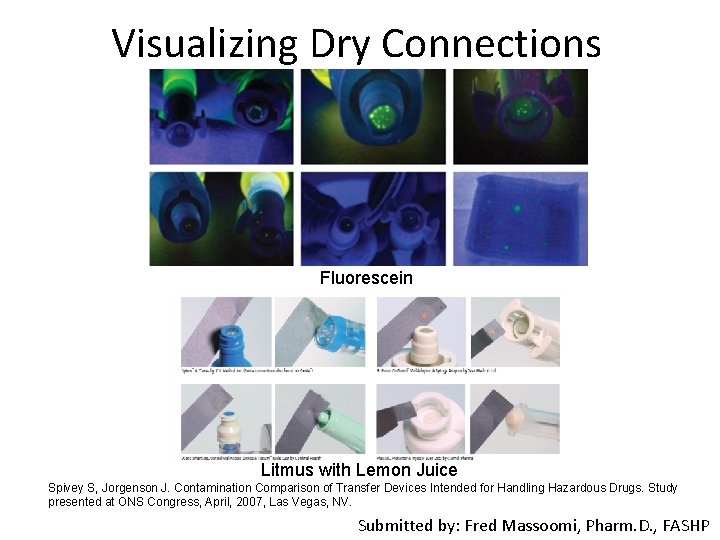
Visualizing Dry Connections Fluorescein Litmus with Lemon Juice Spivey S, Jorgenson J. Contamination Comparison of Transfer Devices Intended for Handling Hazardous Drugs. Study presented at ONS Congress, April, 2007, Las Vegas, NV. Submitted by: Fred Massoomi, Pharm. D. , FASHP
Opening the CSTD Do they really contain vapors? Phaseal Vialshield Marker: Cyclophosphamide Chemolock Equashield Submitted by: Fred Massoomi, Pharm. D. , FASHP
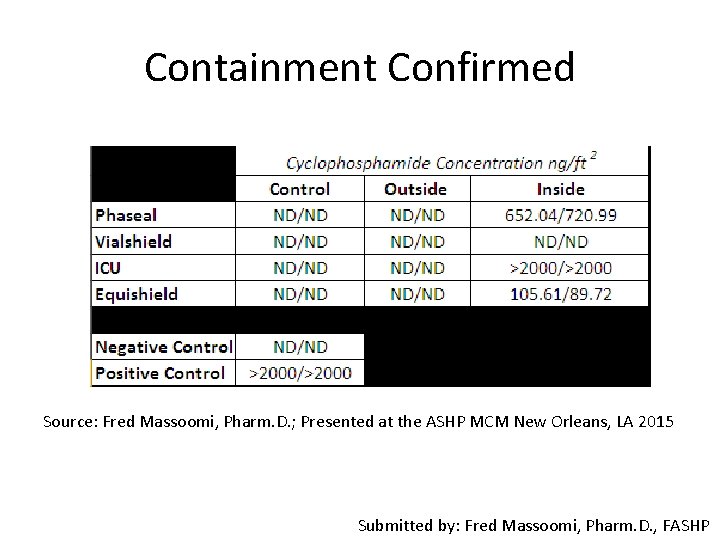
Containment Confirmed Source: Fred Massoomi, Pharm. D. ; Presented at the ASHP MCM New Orleans, LA 2015 Submitted by: Fred Massoomi, Pharm. D. , FASHP

Final Thoughts • Once a test is decided upon where is FDA? • Most tests published are outdated. • How does the consumer interpret results? – Especially conflicting results • Can we just agree that CSTDs are better than a needle and syringe? • Continue to strive to no human manipulation with these products. Submitted by: Fred Massoomi, Pharm. D. , FASHP

Why We Need to Get This Right! Eur J C lin Onc -1 79; Lancet 19 50 8128): 12 June 9: 1( 2 -6 ; 4(1): 7 91 tice 19 c a r P m J Phar AJHP 1 BMJ Qual Saf ol 1986 ; 22(12) published onlin : 1489 -9 e August 16, 2 993; June 9: 1( 8128 5 011 J NCI 19 ): 125 0 93; 85: 1 -1 089 -90 Scand J Work Environ Health 1994; 20: 22 -6 NEJM 1985; 313(19): 1173 -8 -4 1 4; 14(6): 83 8 9 1 d e M ust NZ J A J Occup Environ Med 1997; 39(6): 574 -80 Submitted by: Fred Massoomi, Pharm. D. , FASHP

NIOSH Town Hall Meeting CSTD Testing Firouzan ‘Fred’ Massoomi, Pharm. D. , FASHP Pharmacist 5618 Nicholas St. Omaha, NE 68132 massoomi@cox. net Submitted by: Fred Massoomi, Pharm. D. , FASHP
- Slides: 15