NIHR Governance Systems Regimantas Pestininkas Deputy Research Governance










- Slides: 15

NIHR Governance Systems Regimantas Pestininkas Deputy Research Governance Manager 1 July 2010 West Midlands (South) Comprehensive Local Research Network

Government’s Ambition • Making the NHS a world-class environment for research • Mission: “to create a health research system in which the NHS supports outstanding individuals, working in world-class facilities, conducting leading-edge research, focused on the needs of patients and the public” • Developed with input from government departments, industry, universities and NHS organisations West Midlands (South) Comprehensive Local Research Network

West Midlands (South) Comprehensive Local Research Network

Research Governance • A broad range of regulations, principles and standards of good practice that exist to achieve and continuously improve research quality across all aspects of healthcare in the UK and worldwide • Managing risks & ensuring public confidence • Often viewed as bureaucratic West Midlands (South) Comprehensive Local Research Network

Research Governance Initiatives • Improve the quality, speed and coordination of clinical research by removing the barriers to research in the NHS. Strengthen and streamline systems for research management and governance – Integrated Research Application System – NIHR CSP – Research Passports West Midlands (South) Comprehensive Local Research Network

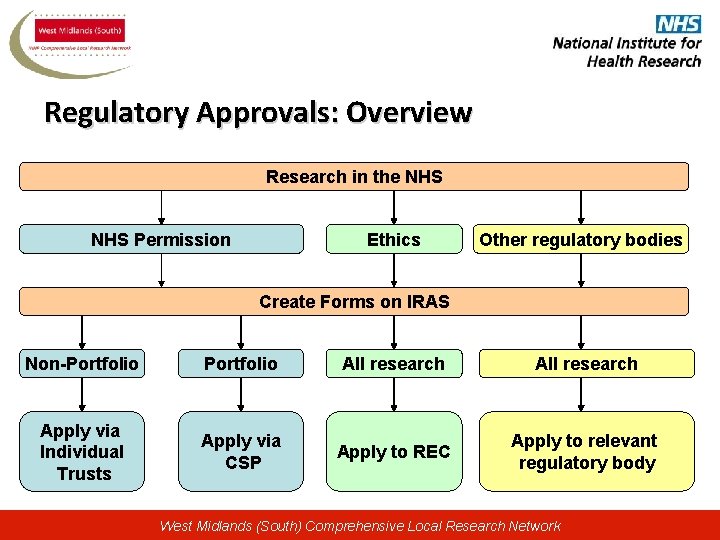
Regulatory Approvals: Overview Research in the NHS Permission Ethics Other regulatory bodies Create Forms on IRAS Non-Portfolio All research Apply via Individual Trusts Apply via CSP Apply to REC Apply to relevant regulatory body West Midlands (South) Comprehensive Local Research Network

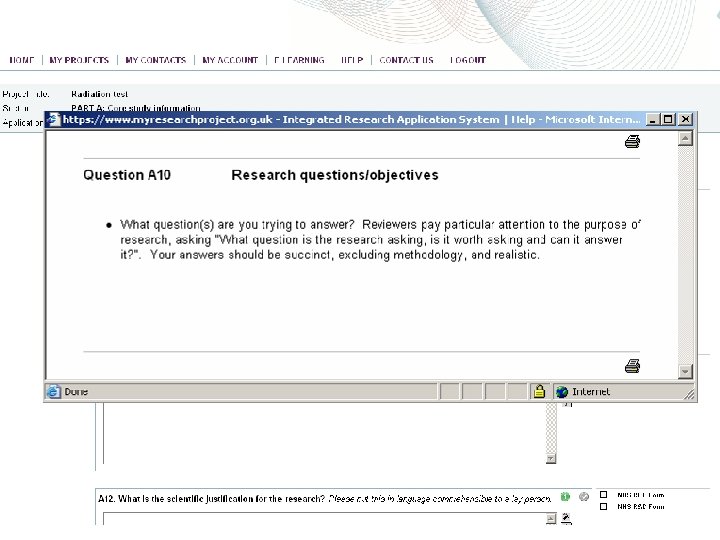
Integrated Research Application System (IRAS) • https: //www. myresearchproject. org. uk • IRAS is a dataset, not a form • Once dataset is created, multiple forms can be populated very easily (REC, R&D, MHRA, NIGB, GTAC, etc) • Data is only entered once! • Ethics and R&D are related, but independent processes; however, they both start through IRAS West Midlands (South) Comprehensive Local Research Network




Coordinated System for gaining NHS Permission (CSP) • A consistent, standardised process for gaining NHS permission • A system run through CSP Unit and CLRNs to ensure a coordinated approach with local input • A single point to which researchers need to apply to gain NHS permission • Distinction between global and local checks – removing duplication West Midlands (South) Comprehensive Local Research Network

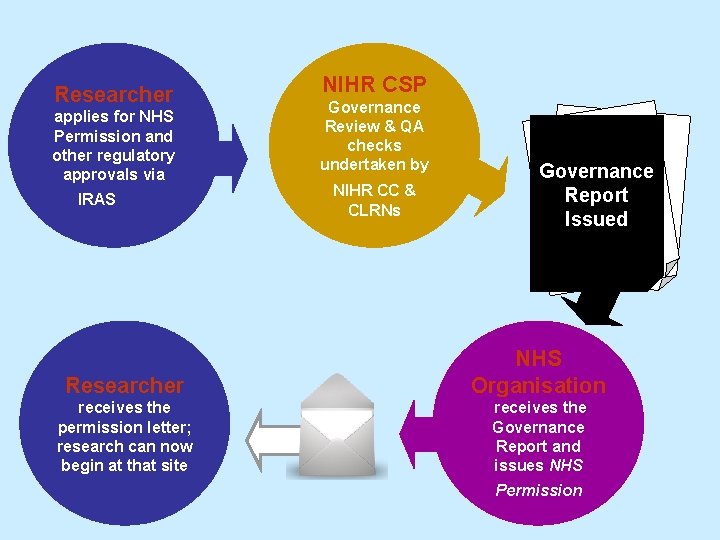
Researcher applies for NHS Permission and other regulatory approvals via IRAS NIHR CSP Governance Review & QA checks undertaken by NIHR CC & CLRNs Governance Report Issued Researcher NHS Organisation receives the permission letter; research can now begin at that site receives the Governance Report and issues NHS Permission

Research Passport Scheme • Allows sharing of pre-engagement information about a researcher with relevant NHS organisations via a Research Passport • Only ONE set of checks are performed on a researcher • Clarifies NHS contractual arrangements for researchers • IMPORTANT: The Research Passport does not remove the need for honorary contracts; it allows them to be issued quicker West Midlands (South) Comprehensive Local Research Network

Future Developments • R&D Management Information System (RDMIS) – – For information input, retrieval and dissemination Sharing of information between partners Easy-to-follow guidance on permissions & conducting research Progress monitoring (recruitment & permissions) • Research Support Services – National framework for research support services – Proportionate risk-based interpretation of policies and rules – Primary focus – facilitating NIHR research, Secondary – facilitating other health research in the NHS West Midlands (South) Comprehensive Local Research Network

Further Information • • www. nihr. ac. uk www. myresearchproject. org. uk www. ukcrn. org. uk CSP: – – National: crncc. csp@nihr. ac. uk BBC CLRN: cspbbcclrn@uhb. nhs. uk WM (N) CLRN: ns-pct. CSP-WMNCLRN@nhs. net WM (S) CLRN: uhc-tr. wmsclrn@nhs. net West Midlands (South) Comprehensive Local Research Network
 Prospero registration
Prospero registration Nihr learn
Nihr learn Viairas
Viairas Nihr advanced fellowship
Nihr advanced fellowship Central commissioning facility research management system
Central commissioning facility research management system Nihr brc
Nihr brc Matt bown
Matt bown Nihr csp
Nihr csp Deputy stowers
Deputy stowers Manifesto for head girl
Manifesto for head girl Onondaga county sheriff civil division
Onondaga county sheriff civil division Confused deputy
Confused deputy Deputy medical director
Deputy medical director Which ics functional area arranges
Which ics functional area arranges Deputy jody hull
Deputy jody hull Eft deputy chief
Eft deputy chief