NIH Grants Strategies to Get Funded Silvia da






































- Slides: 59

NIH Grants: Strategies to Get Funded Silvia da Costa, Ph. D. Director of Faculty Research Relations Office of Research

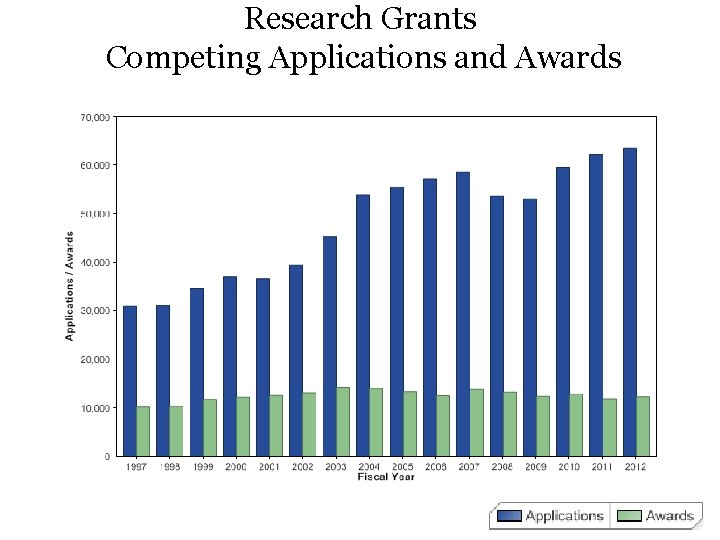
Research Grants Competing Applications and Awards

Strategies to Improve Your Competitiveness Research to address the needs of the funding institute CRISP Re. PORTER Early Stage Investigator Choosing the right study section Grant Sections

Strategies to Improve Your Competitiveness Research to address the needs of the funding institute

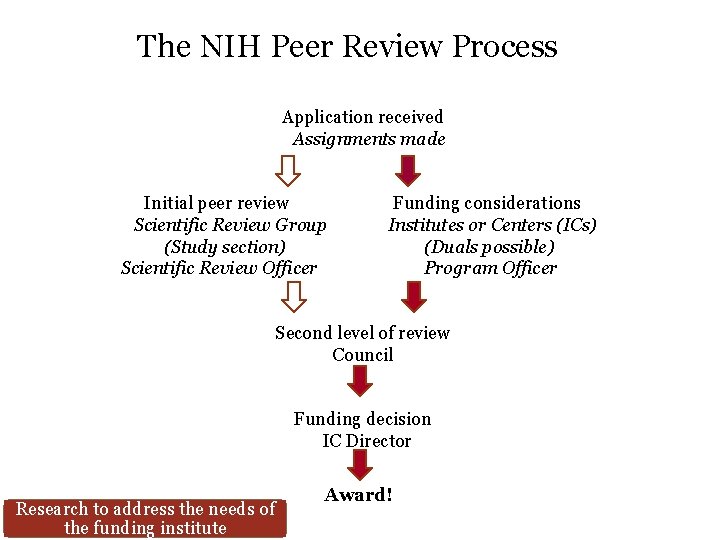
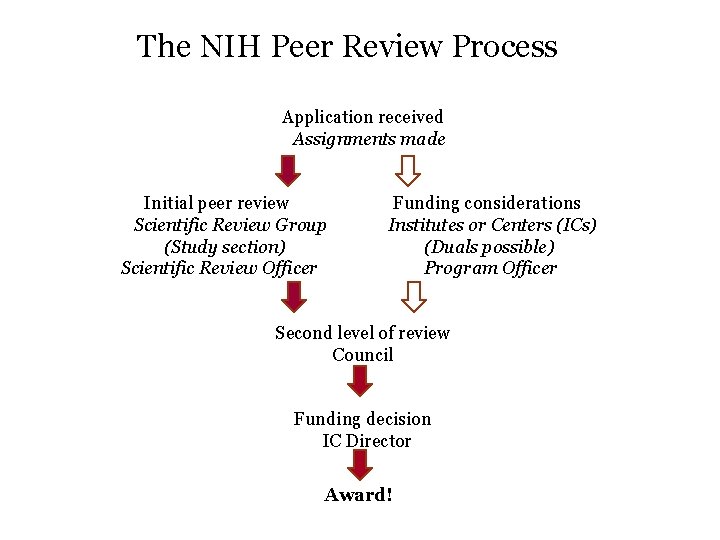
The NIH Peer Review Process Application received Assignments made Initial peer review Funding considerations Scientific Review Group Institutes or Centers (ICs) (Study section) (Duals possible) Scientific Review Officer Program Officer Second level of review Council Funding decision IC Director Research to address the needs of the funding institute Award!

Strategies to Improve Your Competitiveness The NIH is not interested in funding good science The NIH is interested in funding good science that meets the needs of the funding institute “Small business” mentality Research to address the needs of the funding institute

Strategies to Improve Your Competitiveness To which Institute should you submit your grant? Research to address the needs of the funding institute

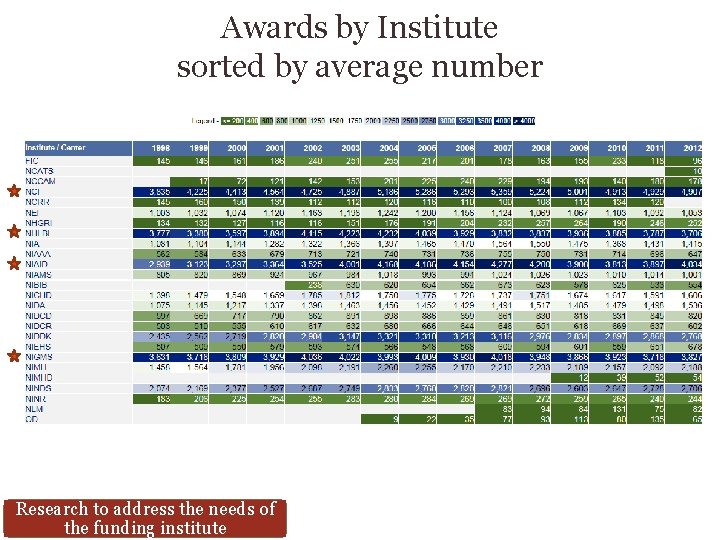
Awards by Institute sorted by average number Research to address the needs of the funding institute

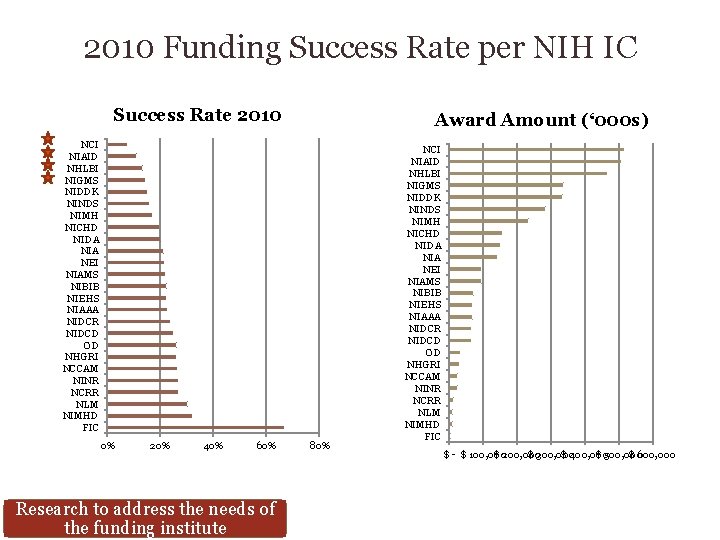
2010 Funding Success Rate per NIH IC Success Rate 2010 Award Amount (‘ 000 s) NCI NIAID NHLBI NIGMS NIDDK NINDS NIMH NICHD NIDA NIA NEI NIAMS NIBIB NIEHS NIAAA NIDCR NIDCD OD NHGRI NCCAM NINR NCRR NLM NIMHD FIC 0% 20% 40% 60% Research to address the needs of the funding institute 80% NCI NIAID NHLBI NIGMS NIDDK NINDS NIMH NICHD NIDA NIA NEI NIAMS NIBIB NIEHS NIAAA NIDCR NIDCD OD NHGRI NCCAM NINR NCRR NLM NIMHD FIC $ - $ 100, 000 $ 200, 000 $ 300, 000 $ 400, 000 $ 500, 000 $ 600, 000

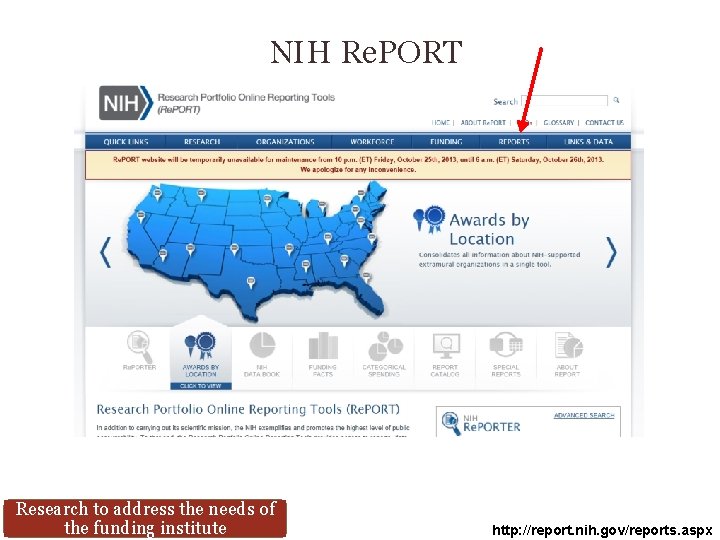
NIH Re. PORT Research to address the needs of the funding institute http: //report. nih. gov/reports. aspx

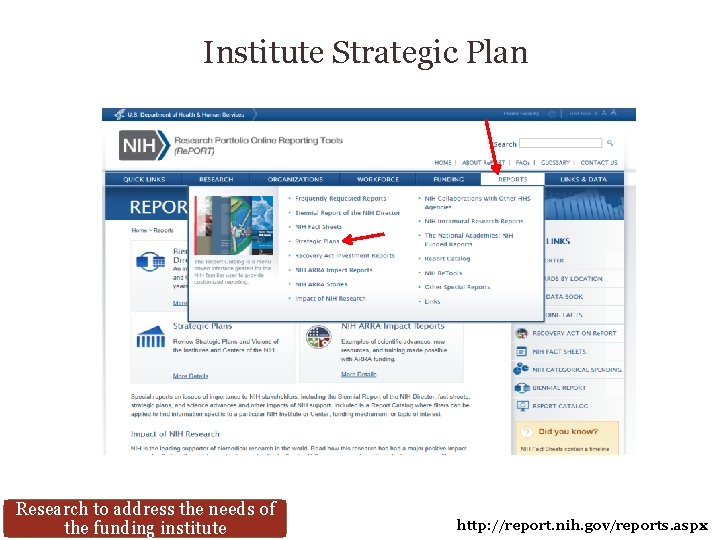
Institute Strategic Plan Research to address the needs of the funding institute http: //report. nih. gov/reports. aspx

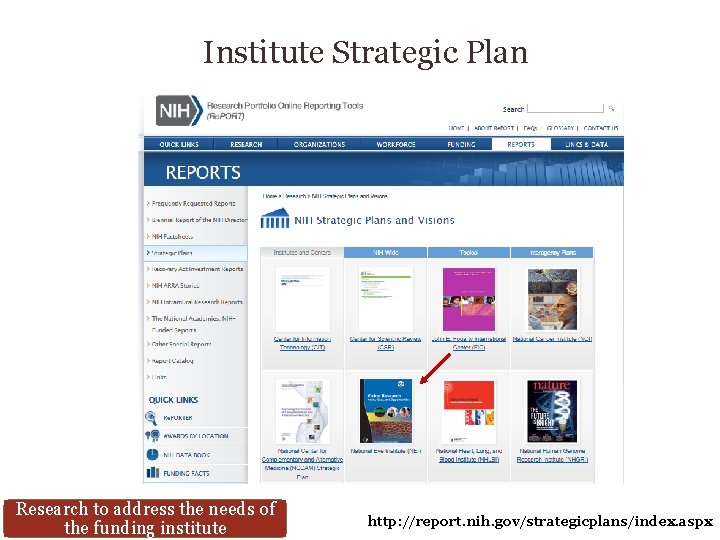
Institute Strategic Plan Research to address the needs of the funding institute http: //report. nih. gov/strategicplans/index. aspx

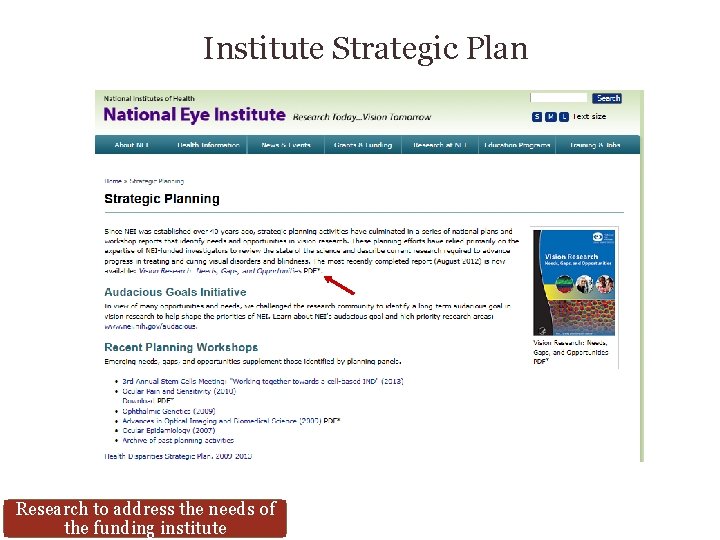
Institute Strategic Plan Research to address the needs of the funding institute

Institute Strategic Plan Research to address the needs of the funding institute

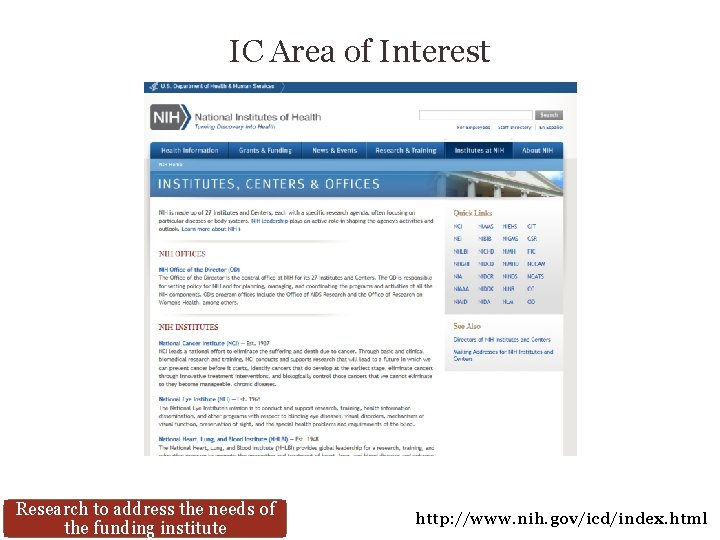
IC Area of Interest Research to address the needs of the funding institute http: //www. nih. gov/icd/index. html

Any Questions Research to address the needs of the funding institute

The NIH Peer Review Process Application received Assignments made Initial peer review Funding considerations Scientific Review Group Institutes or Centers (ICs) (Study section) (Duals possible) Scientific Review Officer Program Officer Second level of review Council Funding decision IC Director Award!

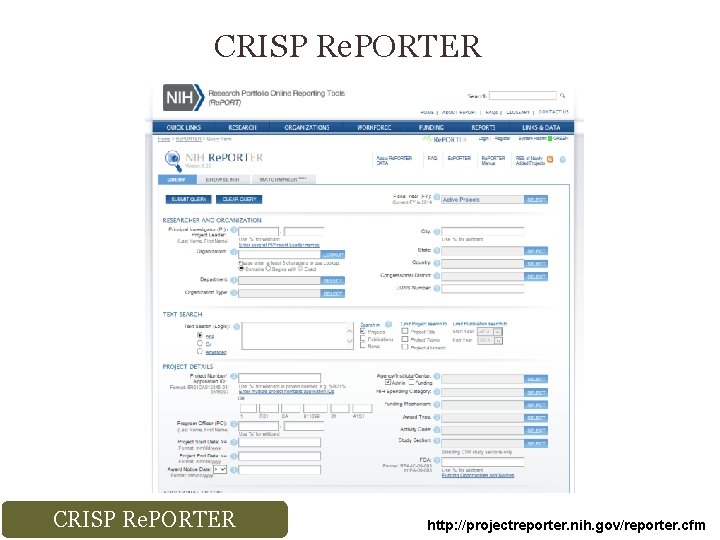
Strategies to Improve Your Competitiveness CRISP Re. PORTER

CRISP Re. PORTER http: //projectreporter. nih. gov/reporter. cfm

CRISP Re. PORTER Keyword “Cancer”, first few pages of search… NCI NIBIB NIA NIGMS NIMHD NINR NHGRI NIAMS NCCAM NIEHS NIAID NCATS OD National Cancer Institute National Institute of Biomedical Imaging and Bioengineering National Institute on Aging National Institute of General Medical Sciences National Institute on Minority Health and Health Disparities National Institute of Nursing Research National Human Genome Research Institute National Institute of Arthritis and Musculoskeletal and Skin Diseases National Center for Complementary and Alternative Medicine National Institute of Environmental Health Sciences National Institute of Allergy and Infectious Diseases National Center for Advancing Translational Sciences Office of the Director CRISP Re. PORTER http: //projectreporter. nih. gov/reporter. cfm

Strategies to Improve Your Competitiveness Choosing the right study section

Strategies to Improve Your Competitiveness Who will be reviewing your grant? Identifying potential members of your Scientific Review Group Choosing the right study section

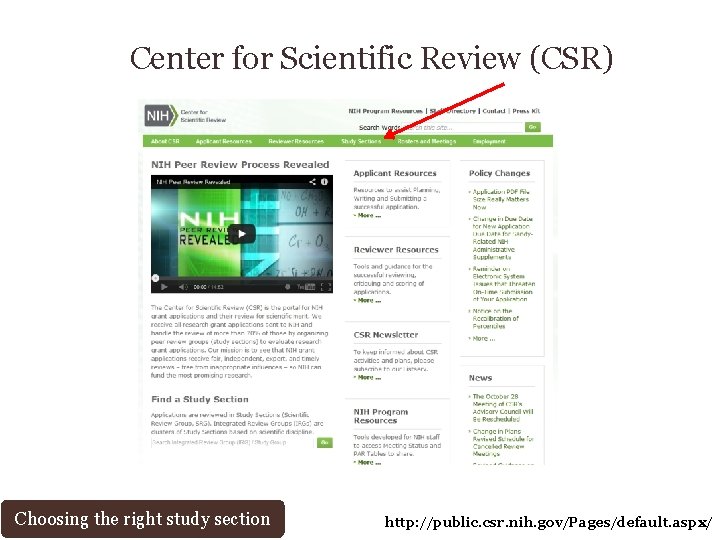
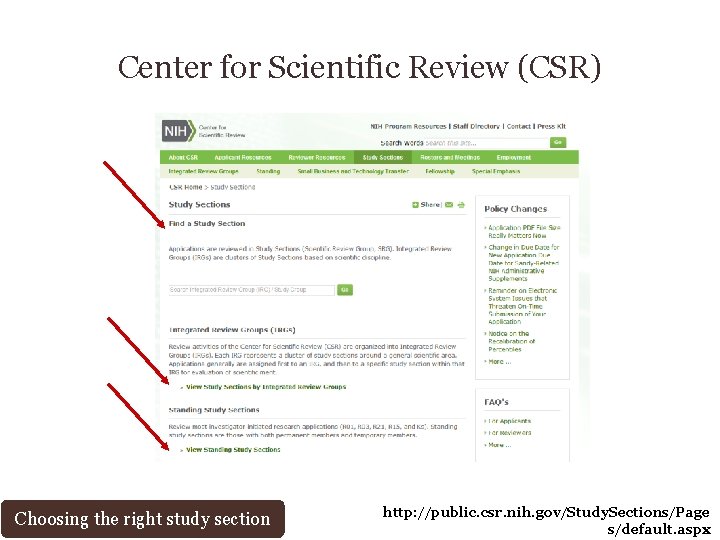
Center for Scientific Review (CSR) Choosing the right study section http: //public. csr. nih. gov/Pages/default. aspx/

Center for Scientific Review (CSR) Choosing the right study section http: //public. csr. nih. gov/Study. Sections/Page s/default. aspx

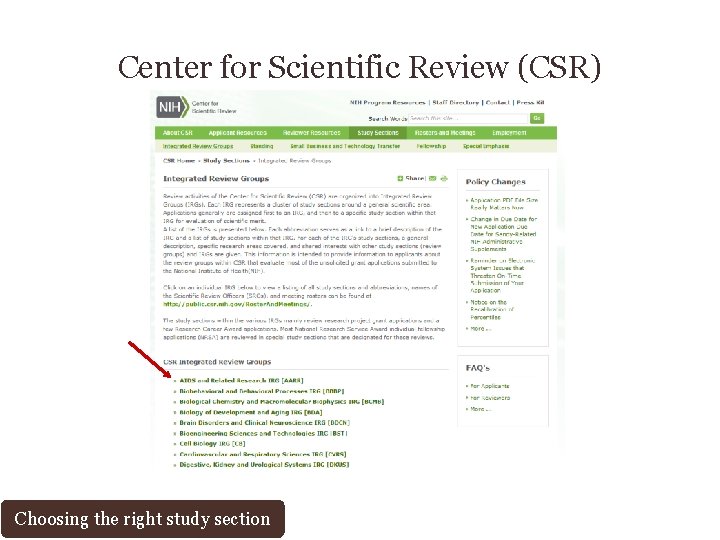
Center for Scientific Review (CSR) Choosing the right study section

Center for Scientific Review (CSR) Choosing the right study section

Any Questions Choosing the right study section

Strategies to Improve Your Competitiveness Early Stage Investigator

NIH Priority: Continued Focus on New Investigators • New Investigator is an NIH research grant applicant who has not yet competed successfully for a substantial, NIH research grant. • Example: a PI who has previously received a competing NIH R 01 research grant is no longer considered a New Investigator. However, a PD/PI who has received a small grant (R 03) or an Exploratory, Developmental Research Grant Award (R 21) retains his or her status as a New Investigator. Early Stage Investigator http: //grants. nih. gov/grants/new_investigators/inve stigator_policies_faqs. htm#2649

NIH Priority: Continued Focus on New Investigators • Early Stage Investigators: ESIs are New Investigators who are within 10 years of completing their terminal research degree or within 10 years of completing their medical residency at the time they apply for R 01 grants. Early Stage Investigator http: //grants. nih. gov/grants/new_investigators/inve stigator_policies_faqs. htm#2649

Funding Policy for NIs & ESIs • Applications from ESIs, like those from all New Investigators, are given special consideration during peer review and at the time of funding. • Peer reviewers are instructed to focus more on the proposed approach than on the track record, and to expect less preliminary information than might be provided by an established investigator. • Applications will be clustered during initial peer review to the extent possible. Early Stage Investigator

Special Programs for NIs & ESIs • Pathway to Independence Award (K 99 -R 00) provides support as a postdoctoral scholar transitions from a training position to a faculty position • Director’s New Innovator Award (DP 2) provides support to highly innovative research approaches Early Stage Investigator http: //grants. nih. gov/grants/new_investigators/inve stigator_policies_faqs. htm#2649

How does the NIH Recognize NIs & ESIs? NI and ESI status is determined automatically by the functionality built into e. RA Commons, based on the investigator’s record of receiving NIH grants and the date of their terminal degree and/or completion of medical residency. Make sure you are correctly designated as an ESI Verify your degree completion date in your NIH Commons Profile (e. RA Commons) Early Stage Investigator http: //grants. nih. gov/grants/new_investigators/inve stigator_policies_faqs. htm#2649

Loss of ESI Status applies only to R 01 s If you are applying for an R 01 with another non-ESI, the proposal will not be reviewed as an ESI application. If awarded, you will lose your ESI status. Need to balance use of experienced collaborator with loss of ESI status. Early Stage Investigator

Strategies to Improve Your Competitiveness Grant sections

Good Grantsmanship Grant writing is a learned skill! Grant sections

Approach: Restructured Research Plan Previous Application New Application Background and Significance a. Significance b. Innovation Research Design and c. Approach Methods • Preliminary Studies Preliminary for New Applications Studies/Progress Report • Progress Report for Renewal/Revision Grant sections

Important to differentiate between the two! Significance (1/2 page) • Does the project address an important problem or a critical barrier to progress in the field? • If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? • How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field? Innovation (1/2 page) • Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? • Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? • Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed? Grant sections

Biographical Sketch • Personal Statement –what experience and qualifications make the applicant particularly wellsuited for the project. • Limited to 4 pages (per person) • Publications limited to 15 – 5 most recent – 5 best – 5 most relevant to the application Grant sections

Biosketch: Include the PMCID Example Varmus H, Klausner R, Zerhouni E, Acharya T, Daar A, Singer P. 2003. PUBLIC HEALTH: Grand Challenges in Global Health. Science 302(5644): 398– 399. PMCID: PMC 243493 Grant sections http: //publicaccess. nih. gov/citation_methods. htm

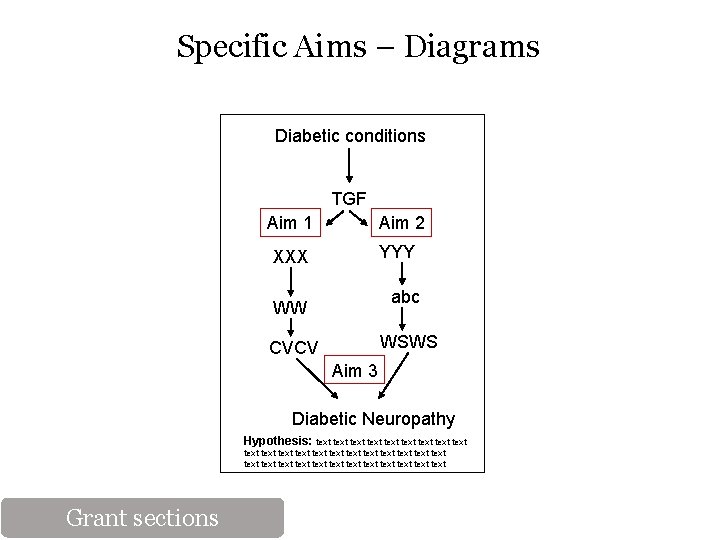
Specific Aims Page - Outline Background information Relevance (medical/clinical) Gap in knowledge/Current knowledge Long-term goal (of your lab) Objective of the proposal Hypothesis - Basis for hypothesis Rational for study Specific Aims Hypothesis How it will be tested Expected Results Why proposal is innovative Significance PI / Environment Positive Impact “Payoff” for the Institute/Foundation Grant sections

Specific Aims Page – Target Audience Grant sections

Specific Aims – Diagrams Diabetic conditions TGF Aim 1 Aim 2 XXX YYY abc WW WSWS CVCV Aim 3 Diabetic Neuropathy Hypothesis: text text text text text text text text text Grant sections

Specific Aims Page Your job is to make the reviewer’s work easier! Background information Relevance (medical/clinical) Gap in knowledge/Current knowledge Long-term goal (of your lab) Objective of the proposal Hypothesis - Basis for hypothesis Rational for study Specific Aims Hypothesis How it will be tested Expected Results Why proposal is innovative Significance PI / Environment Positive Impact “Payoff” for the Institute/Foundation Grant sections What is not known is … It is relevant because… The objective of the proposal is. . The rational is based on the need to… This proposal is innovative because… The research is significant because. . It will have a positive impact due to… Our unique research environment specializing in XYZ will assure the success of the proposed project… It helps the XX institute fulfill it’s mission towards… or is in line with the goals of the institute in that…

Specific Aims Page Abstract Grant sections

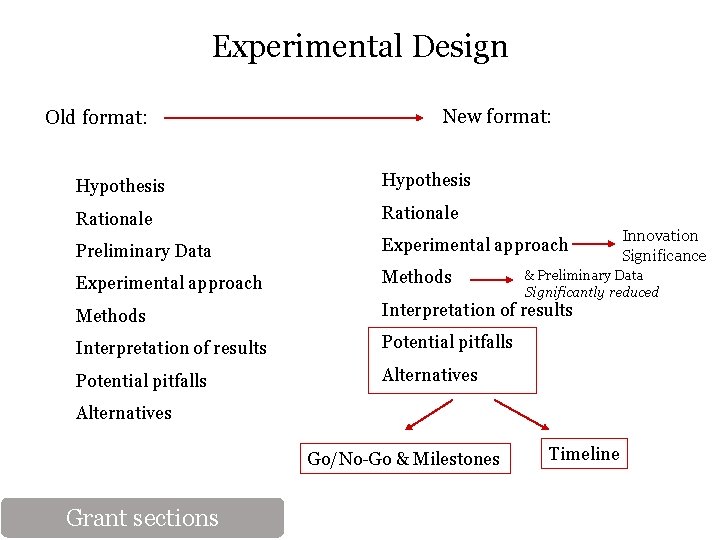
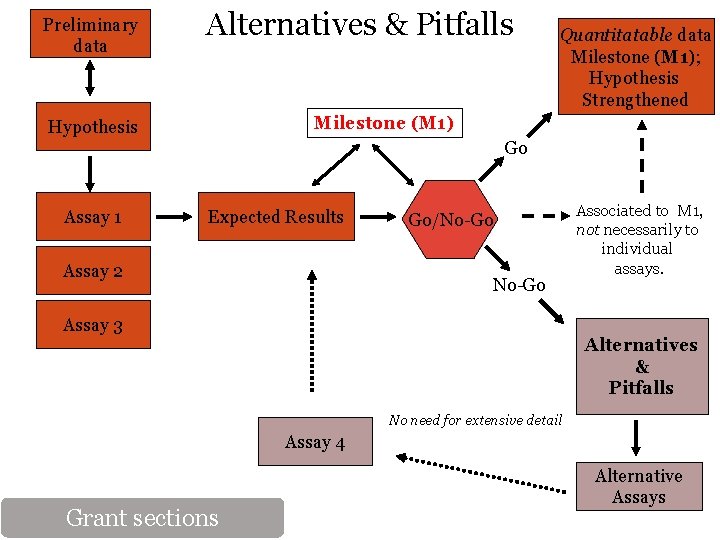
Experimental Design Old format: New format: Hypothesis Rationale Preliminary Data Experimental approach Methods Interpretation of results Potential pitfalls Alternatives & Preliminary Data Significantly reduced Alternatives Go/No-Go & Milestones Grant sections Innovation Significance Timeline

Preliminary data Alternatives & Pitfalls Quantitatable data Milestone (M 1); Hypothesis Strengthened Milestone (M 1) Hypothesis Go Assay 1 Expected Results Assay 2 Go/No-Go Assay 3 Associated to M 1, not necessarily to individual assays. Alternatives & Pitfalls No need for extensive detail Assay 4 Grant sections Alternative Assays

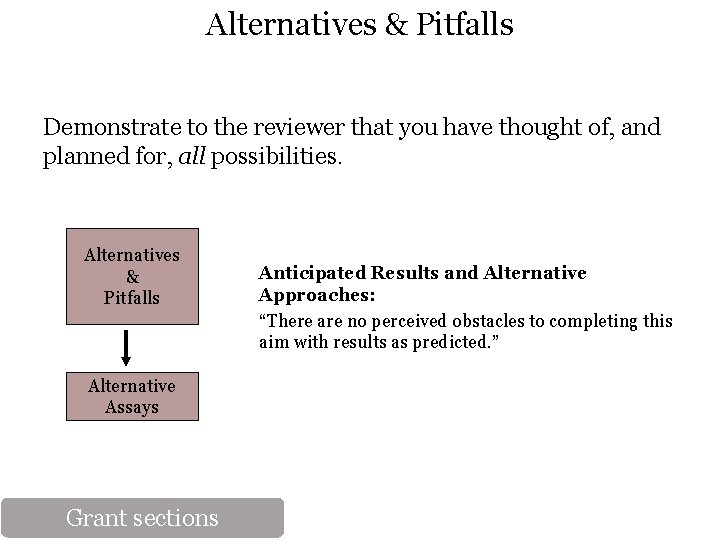
Alternatives & Pitfalls Demonstrate to the reviewer that you have thought of, and planned for, all possibilities. Alternatives & Pitfalls Alternative Assays Grant sections Anticipated Results and Alternative Approaches: “There are no perceived obstacles to completing this aim with results as predicted. ”

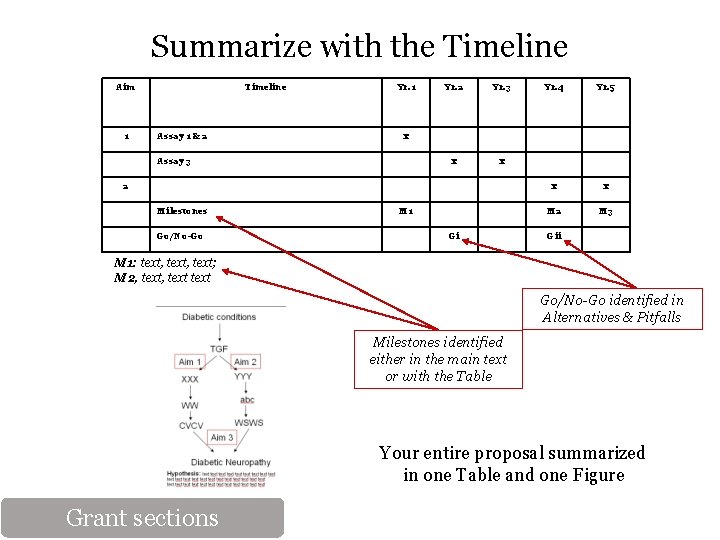
Summarize with the Timeline Aim 1 Timeline Assay 1 & 2 Yr. 1 Yr. 2 Yr. 3 x x 2 Go/No-Go Yr. 5 x x M 2 M 3 x Assay 3 Milestones Yr. 4 M 1 Gi Gii M 1: text, text; M 2, text Go/No-Go identified in Alternatives & Pitfalls Milestones identified either in the main text or with the Table Your entire proposal summarized in one Table and one Figure Grant sections

Grant Proposal Cover Letter Application title FOA # and title Request: • • • Place SRG & IC review requests on separate lines Place positive & negative requests on separate lines Include name of IC or SRG, followed by a dash and acronym Provide explanations for each request in a separate paragraph You can ask for a specific study section but it is not necessarily guaranteed… • Check e. RA Commons regularly to see confirm to where it was assigned. • Contact the PO immediately if it was not assigned to the section you wanted - they usually will try to accommodate your request Choosing the right study section

Response to Reviewers Q: What if you know that you are “Right” and the reviewers are “Wrong”, is it appropriate to argue your position in your resubmission? A: NO! Never be Argumentative ! Never be Abrasive ! Do not do long term damage to yourself Always address all comments and critiques Thank the reviewer for their effort Remind them of the good comments Choosing the right study section Grant sections

Response to Reviewers How to shoot yourself in the foot… The reviewer’s comments regarding the proposed mode of action of XXX are frankly astonishing and somewhat disturbing as they suggest a view biased in favor of the more conventional mode of action for antibody. Clearly this reviewer is not familiar with the anti-inflammatory properties of XXX and apparently did not read the background sections on ‘Antibody prophylaxis and therapy’ (section 3. 3) and ‘Antiinflammatory Activity of XXX’ (section 3. 4) in which XXX mechanisms of action were discussed. Choosing the right study section Grant sections

Any Questions Grant sections

Word Reduction & Editing Suggestions Early Stage Investigator

Methods – Keep it Brief The power of parenthesis… A total of 1 x 107 cells in 0. 4 ml of serum-free RPMI 1640 medium was transfected with 2 g of the reporter plasmid, 0. 5 g of the Renilla luciferase control vector (p. RL-TK; Promega), and 30 g of the expression vector by electroporation (250 V and 950 F). Following electroporation, cells were incubated for 10 minutes at room temperature and then transferred into growth 10 ml of medium and cultured at 37 C and 5% CO 2 for 40– 48 hours. A total of 1 x 107 cells in 0. 4 ml of serum-free RPMI 1640 medium was transfected with 2 g of the reporter plasmid, 0. 5 g of the Renilla luciferase control vector (p. RL-TK; Promega), and 30 g of the expression vector by electroporation (250 V and 950 F). Following electroporation, cells were incubated for 10 minutes at room temperature and then transferred into growth 10 ml of medium and cultured at 37°C and 5% CO 2 for 40– 48 hours. Cells (1 x 107 in 0. 4 ml of serum-free RPMI 1640 medium) were transfected with the reporter plasmid (2 g), Renilla luciferase control (0. 5 g, p. RL-TK; Promega), and expression vectors (30 g), by electroporation (250 V, 950 F), incubated (10 min, room temperature), transferred into growth medium (10 ml) and cultured (37 C, 5% CO 2, 40 - 48 h). 78 to 58 words…

Methods – Keep it Brief Cells (1 x 107 in 0. 4 ml of serum-free RPMI 1640 medium) will be transfected with the reporter plasmid (2 g), Renilla luciferase control (0. 5 g, p. RL-TK; Promega), and expression vectors (30 g), by electroporation (250 V, 950 F), incubated (10 min, room temperature), transferred into growth medium (10 ml) and cultured (37 C, 5% CO 2, 40 - 48 h). Cells will be transfected by electroporation with the reporter plasmid, Renilla luciferase control and expression vector, then transferred into growth medium and cultured (40 - 48 h). 58 to 23 words…

Figure Legends… Keep it brief Figure 2. (A) The spots of proteins in the 2 -D gels: DR 0099, DR 2340 and DRA 0346: Ss. B, Rec. A and Ppr. A, respectively. (B) The spots of proteins in the 2 -D gels: DR 0307 and DR 1082: elongation factor G and lightrepressed protein A, respectively. (C) The spots of proteins in the 2 D gels: DR 1473 and DR 2128: phage shock protein A and DNAdirected RNA polymerase alpha subunit, respectively. (D) Relative protein expression levels of proteins. Figure 2. Protein spots in 2 -D gels for (A) DR 0099, DR 2340 and DRA 0346: Ss. B, Rec. A and Ppr. A, respectively; (B) DR 0307 and DR 1082: elongation factor G and light-repressed protein A, respectively and (C) DR 1473 and DR 2128: phage shock protein A and DNA-directed RNA polymerase alpha subunit, respectively. (D) Relative protein expression levels Protein expression was calculated as described in experimental procedures. (see Experimental Procedures) The values are the mean ± standard deviation (mean ± SD) of four independent experiments repeated twice each. (n=4, in duplicate) 94 to 58 words…

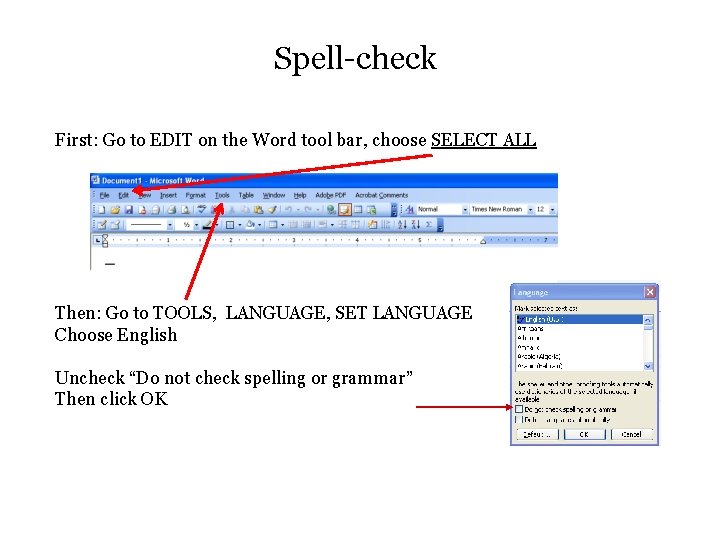
Spell-check First: Go to EDIT on the Word tool bar, choose SELECT ALL Then: Go to TOOLS, LANGUAGE, SET LANGUAGE Choose English Uncheck “Do not check spelling or grammar” Then click OK

“What is written without effort is, in general, read without pleasure. ” Samuel Johnson Question marks from Stock images
 Get on / get off transport
Get on / get off transport One direction metaphors
One direction metaphors This project is funded by the european union
This project is funded by the european union Co-funded by the erasmus+ programme of the european union
Co-funded by the erasmus+ programme of the european union This project is funded by the european union
This project is funded by the european union This project is funded by the european union
This project is funded by the european union Action research
Action research This project is funded by the european union
This project is funded by the european union This project has been funded by contributions
This project has been funded by contributions Co-funded by the erasmus+ programme of the european union
Co-funded by the erasmus+ programme of the european union This project is funded by the european union
This project is funded by the european union This project has been funded by
This project has been funded by Self-funded pso
Self-funded pso Non-governmental health agencies are funded primarily by
Non-governmental health agencies are funded primarily by This project is co-funded by the european union
This project is co-funded by the european union This project has been funded by
This project has been funded by National general benefits solutions
National general benefits solutions This project is funded by the european union
This project is funded by the european union Self funded vs fully insured
Self funded vs fully insured Co-funded by the erasmus+ programme of the european union
Co-funded by the erasmus+ programme of the european union Get up get moving quiz
Get up get moving quiz Selection pseudocode
Selection pseudocode Get up get moving quiz
Get up get moving quiz Get focused get results
Get focused get results Get up get moving quiz
Get up get moving quiz Germer
Germer Orphan products grants program
Orphan products grants program Iu grad grants
Iu grad grants Seta business funding
Seta business funding Nato pdd grants
Nato pdd grants Grants ap gov
Grants ap gov Energy efficiency grants chicago
Energy efficiency grants chicago Peace corps grants
Peace corps grants Grants management system gms
Grants management system gms Hrsa capital grants
Hrsa capital grants Fp&m seta funding
Fp&m seta funding How did land grants and new roads affect brazil?
How did land grants and new roads affect brazil? Arts council national lottery project grants
Arts council national lottery project grants Intergovernmental grants department
Intergovernmental grants department Tdem grants management system
Tdem grants management system Tennair
Tennair Privilege diagram
Privilege diagram Noaa nesdis org chart
Noaa nesdis org chart Hesaa njfams
Hesaa njfams Grants ap gov
Grants ap gov Categorial grants
Categorial grants Digitization grants
Digitization grants Ies research grants
Ies research grants Grants management system gms
Grants management system gms Ken emond british academy
Ken emond british academy Ezfed
Ezfed Hrsa grants management
Hrsa grants management Emd serono grants
Emd serono grants Charlotte lesley
Charlotte lesley Ez fed
Ez fed Nih payment management system
Nih payment management system Sql authorization mechanism grants privileges on
Sql authorization mechanism grants privileges on Grants lick elementary
Grants lick elementary Dep environmental education grants
Dep environmental education grants Fpm seta indicium
Fpm seta indicium