NIH Genomic Data Sharing GDS Policy Kathleen Calzone











- Slides: 20

NIH Genomic Data Sharing (GDS) Policy Kathleen Calzone, Ph. D, RN, APNG, FAAN Anjan Purkayastha, Ph. D February 22, 2016

GDS Scope The GDS Policy applies to all NIH-funded research that generates large-scale human or non-human genomic data as well as the use of these data for subsequent research Large-scale data include data from genome-wide association studies (GWAS) and single nucleotide polymorphism (SNP) arrays, as well as genome sequence, transcriptomic, metagenomic, epigenomic, and gene expression data, irrespective of funding level and funding mechanism

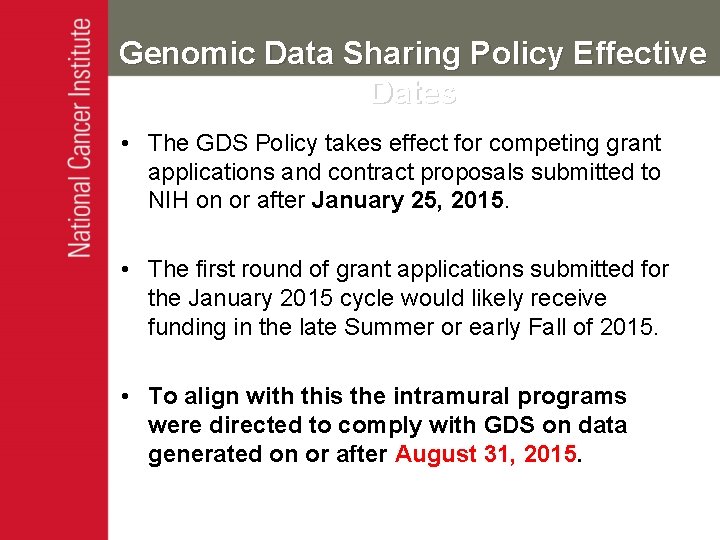
Genomic Data Sharing Policy Effective Dates • The GDS Policy takes effect for competing grant applications and contract proposals submitted to NIH on or after January 25, 2015. • The first round of grant applications submitted for the January 2015 cycle would likely receive funding in the late Summer or early Fall of 2015. • To align with this the intramural programs were directed to comply with GDS on data generated on or after August 31, 2015.

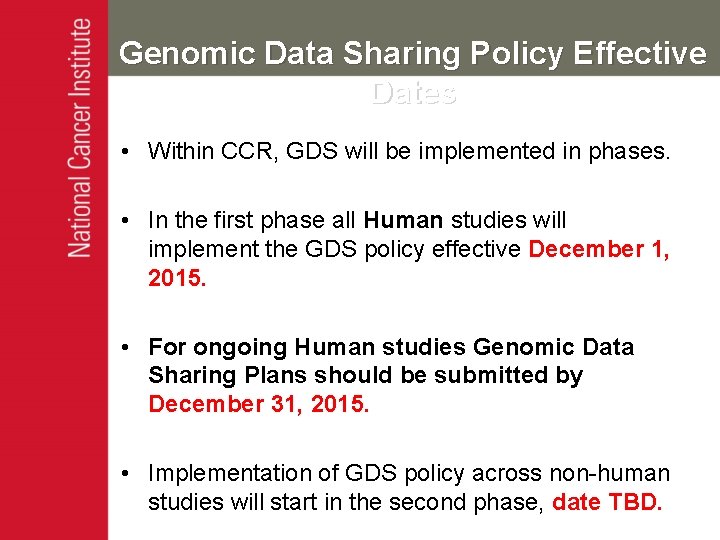
Genomic Data Sharing Policy Effective Dates • Within CCR, GDS will be implemented in phases. • In the first phase all Human studies will implement the GDS policy effective December 1, 2015. • For ongoing Human studies Genomic Data Sharing Plans should be submitted by December 31, 2015. • Implementation of GDS policy across non-human studies will start in the second phase, date TBD.

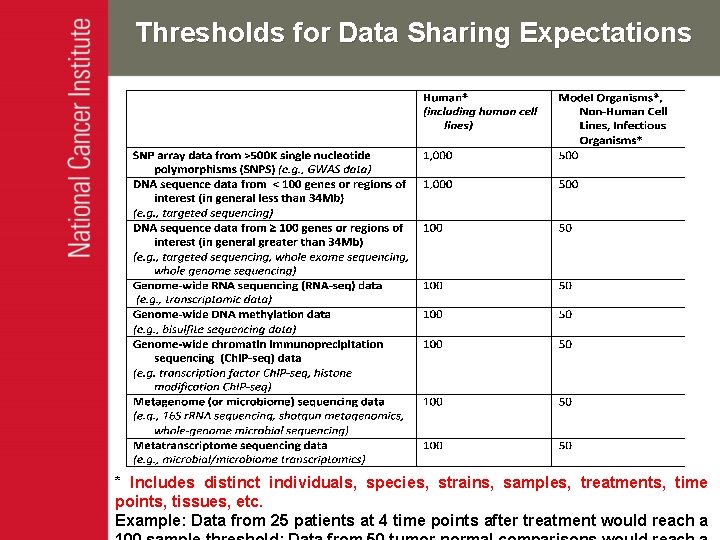
Thresholds for Data Sharing Expectations * Includes distinct individuals, species, strains, samples, treatments, time points, tissues, etc. Example: Data from 25 patients at 4 time points after treatment would reach a

Shareable Data: Additional Criteria Based on NIH IC priorities, GDS policy may apply to smaller data-sets: ü State of science ü NCI’s programmatic priorities ü Utility of data to research community Examples of smaller-scale projects that the NCI anticipates data sharing for (regardless of study design) include, but are not limited to: ü Projects examining rare cancers or rare cancer related outcomes, rare cancer subtypes. ü Projects focusing on under-studied populations. ü Journal(s) may require data sharing before publishing your manuscript

Examples of Research Outside the Scope of the GDS Policy Examples of projects outside the Policy’s scope include, but are not limited to, those that do not meet the criteria in the above examples and involve: X Instrument calibration exercises. X Statistical or technical methods development.

Genomic Data Sharing Plan (GDSP) Purpose of the GDSP • Dataset value for use in secondary analyses • Costs and other resource issues pertaining to data deposition, management, or access needs (e. g. , the availability of appropriate public data repositories or other data sharing mechanisms) • Compliance and utility tracking Clinical Trial • GDSP should be submitted to at the time of IRB review and as approval is being made. § CCR PSO working on study amendments for open protocols Other Research • The GDSP should be submitted prior to data generation. § Ongoing studies must submit a GDSP if data

Institutional Certification (IC) An IC must accompany the submission of all human data to the db. Ga. P at the time of study registration • Explicit informed consent for open access data sharing should be provided by research participants for studies depositing full genome sequence into open access, whether sample or data collection is prospective or retrospective • Assures that the Institutional Review Board (IRB) reviewed the informed consent and protocol to assure that data sharing is appropriate and identified any data use limitations that may exist based on the language found in the consent Clinical Trials • IC memo(s) should be filled immediately following IRB approval Other Research • IC memo(s) should be filled out prior to data generation

Requests for Exceptions to Data Sharing, contd. • In rare cases, NCI will consider requests for an exception to usual data submission expectations. • Submission of genomic data may be precluded by: – international laws; limitations in the original informed consents; concerns about harms to individuals or groups; or other cases where expectations for data submission cannot be met. Exception Requests: • Investigators provide a justification for any data submission exceptions prior to data generation and include an alternative data sharing plan. • The Exception Request form should be signed by – Branch Chief – GPA and/or IRB, if using a consent justification for exception – CCR Scientific Director • Trans-NCI Data Sharing Working Group for a recommendation • NCI Scientific Program Leaders (SPL) for a consensus recommendation

Requests for Exceptions to Data Sharing NCI uses the following criteria to assess exception requests: • • Impact of data sharing compliance on scientific merit Unique resource High value resource Regulatory considerations – Missing consent from patients • Ethical considerations • NIH data sharing exception precedents • Is there an acceptable alternative data-sharing plan (ADSP)? – Impact of ADSP on data re-use – Impact of ADSP on data discoverability – Burden – Feasibility • In all cases where an ADSP is determined to be appropriate, information on how to request access to data and a basic

GDS Policy Infrastructure, Resources for CCR

Developing a Genomic Data Sharing Plan (GDSP) • The GDSP outlines a PI’s plan for generating genomic data: sample size; data type; estimated timeline; repository • Each project/protocol requires a separate GDSP • Each project under an Omnibus protocol needs its own GDSP • A GDSP SOP (SOP-RPS-21) has been developed for CCR • GDSP Submission Process Overview: • PI completes and submits a GDSP to the GPA • The SD, or their delegate, and GPA reviews GDSP

Developing a GDSP: An example The CCR GDS website: http: //bit. ly/CCR_GDS

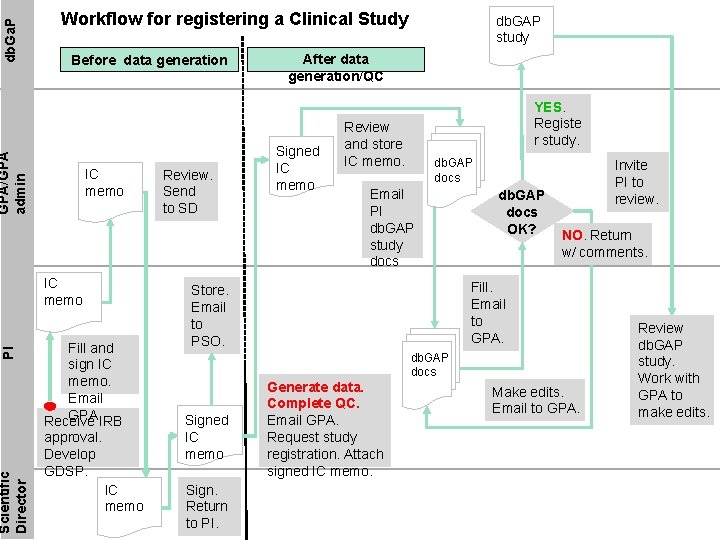
Registering a Clinical Study • Institutional Certification (IC): PIs are responsible for assuring, through an IC, that data have been collected with informed consent from participants and in accordance with federal regulations safeguarding participant privacy. • A Clinical Study Registration SOP has been developed for CCR- RPS-23. • Clinical Study Registration Process Overview: – Before data generation • On IRB approval develop a GDSP and submit to GPA and SD for approval • After GDSP has been accepted, fill IC; sign; submit to GPA • GPA reviews; forwards to SD for review • SD reviews, signs and returns to PI with a copy to GPA • Mail a signed copy of IC to PSO

Registering a Clinical Study contd. • Clinical Study Registration Process Overview: – After data generation/QC/cleaning • Contact GPA with a request to register study in db. Ga. P. Attach a copy of IC. • You must register your study even if you have been granted a data-sharing exception. • GPA will send you: – db. GAP study configuration template – db. GAP Basic Study Information Sheet. • Complete forms and mail back to GPA • GPA will: – Ensure that forms are completely and accurately filled out – Register the study in the db. GAP portal – Invite PI to review the registered study and determine who will be the data submitter – PI or designee as assigned by the PI • Once registration is complete, db. Ga. P will send PI an email invite to review study details

Registering a Clinical Study: An example The CCR GDS website: http: //bit. ly/CCR_GDS

Questions and Answers Genomic Program Administrator (GPA) Kathleen Calzone, Ph. D, RN, APNG, FAAN 301 -435 -0538, calzonek@mail. nih. gov GPA Administrator Anjan Purkayastha, Ph. D 301 -594 -1395, anjan. purkayastha@nih. gov

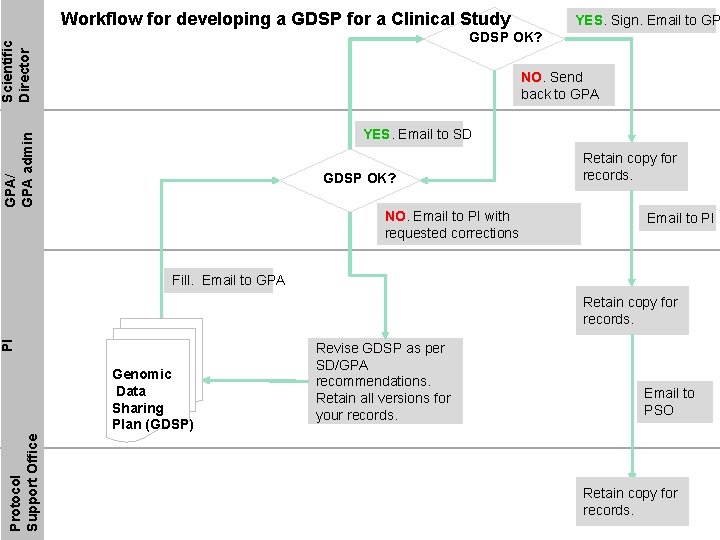
Workflow for developing a GDSP for a Clinical Study YES. Sign. Email to GP Scientific Director GDSP OK? NO. Send back to GPA/ GPA admin YES. Email to SD GDSP OK? NO. Email to PI with requested corrections Retain copy for records. Email to PI Fill. Email to GPA PI Retain copy for records. Protocol Support Office Genomic Data Sharing Plan (GDSP) Revise GDSP as per SD/GPA recommendations. Retain all versions for your records. Email to PSO Retain copy for records.

Before data generation GPA/GPA admin db. Ga. P Workflow for registering a Clinical Study IC memo Scientific Director PI IC memo Fill and sign IC memo. Email GPA. IRB Receive approval. Develop GDSP. IC memo Review. Send to SD db. GAP study After data generation/QC Signed IC memo YES. Registe r study. Review and store IC memo. db. GAP docs Email PI db. GAP study docs db. GAP docs OK? Invite PI to review. NO. Return w/ comments. Fill. Email to GPA. Store. Email to PSO. db. GAP docs Signed IC memo Sign. Return to PI. Generate data. Complete QC. Email GPA. Request study registration. Attach signed IC memo. Make edits. Email to GPA. Review db. GAP study. Work with GPA to make edits.