NIDAS CLINICAL TRIALS NETWORK A SOURCE OF SOCIAL























- Slides: 31

NIDA’S CLINICAL TRIALS NETWORK: A SOURCE OF SOCIAL WORK PRACTICE-RELEVANT KNOWLEDGE Elizabeth Wells School of Social Work University of Washington Dennis Daley Western Psychiatric Institute University of Pittsburgh Supported by Grants # U 10 DA 020036 and U 10 DA 013714 from the National Institute on Drug Abuse

v “…serious gaps of communication exist between the research community and community-based drug treatment programs. ”* *IOM, 1998 – Executive Summary

CTN Mission v. Study behavioral and pharmacological treatments that have high potential for success in community treatment settings v. Get research findings into the hands of clinicians who can make the best use of them – as fast as possible

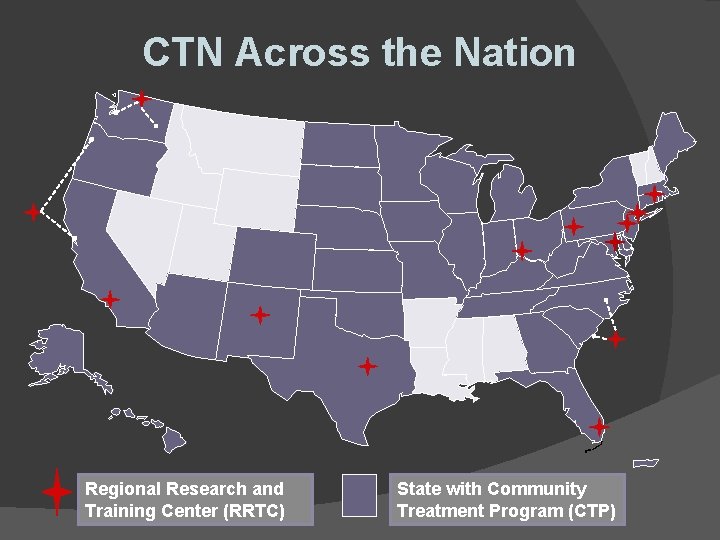
CTN Across the Nation Regional Research and Training Center (RRTC) State with Community Treatment Program (CTP)

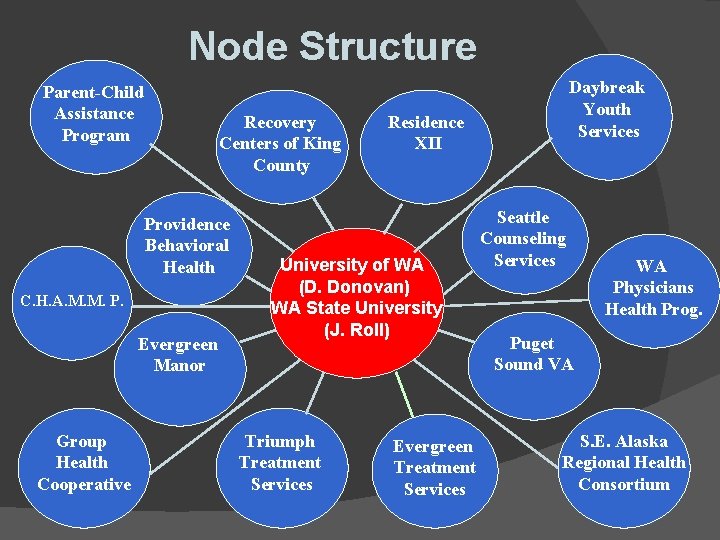
Node Structure Parent-Child Assistance Program Recovery Centers of King County Providence Behavioral Health C. H. A. M. M. P. Evergreen Manor Group Health Cooperative Residence XII University of WA (D. Donovan) WA State University (J. Roll) Triumph Treatment Services Daybreak Youth Services Evergreen Treatment Services Seattle Counseling Services WA Physicians Health Prog. Puget Sound VA S. E. Alaska Regional Health Consortium

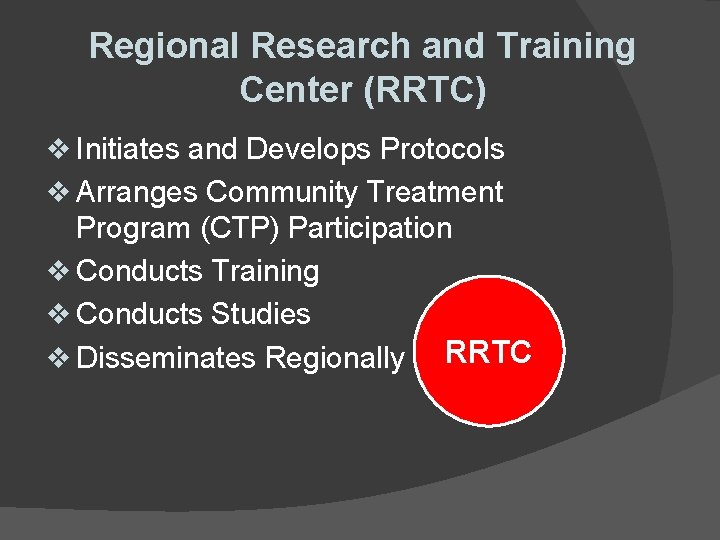
Regional Research and Training Center (RRTC) v Initiates and Develops Protocols v Arranges Community Treatment Program (CTP) Participation v Conducts Training v Conducts Studies v Disseminates Regionally RRTC

Community Treatment Program (CTP) v Equal representation on national CTN Steering Committee v Participates fully in CTN committees v Generates clinically relevant concepts v Provides setting and staffing for CTN studies v Collects data CTP v Potential early adopters

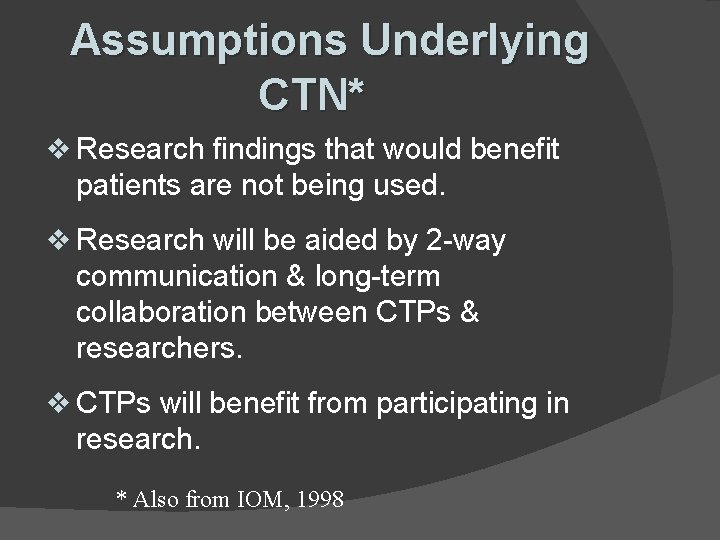
Assumptions Underlying CTN* v Research findings that would benefit patients are not being used. v Research will be aided by 2 -way communication & long-term collaboration between CTPs & researchers. v CTPs will benefit from participating in research. * Also from IOM, 1998

Multi-Site Hybrid Efficacy – Effectiveness* Trials External Validity Internal Validity v Community-based clinics v Heterogeneity of participants v Community clinicians, v Comparison to “treatment -as-usual” v Cost-effectiveness evaluation v Patient & clinician satisfaction v Interest in sustainability v Random assignment v Study protocol & operations manual v Manual-based treatment v Fidelity monitoring v Standard measures v Quality assurance * Carroll, K. M. & Rounsaville, B. J. (2003) Psychiatric Services, 54.

Increasing Role for Social Work in Addiction Treatment v Integration of substance use and mental health treatment (behavioral health) v With health care reform, larger numbers of behavioral health clients served v With health care reform, integration of behavioral health into health care settings, e. g. , primary care

Social Workers’ Multiple Roles in Behavioral Health v. Managers in behavioral health settings v. Providers in health care settings v. Care managers in integrated care v. Policy development and oversight

What does the CTN Offer Social Workers? 1. EBPs - What psychosocial or pharmacological interventions for treating substance use disorders (SUD) are effective? 2. EBPs for adolescents 3. EBPs for HIV prevention in SUD treatment settings 4. Evidence-based for all populations (e. g. , women, racial and ethnic minorities, sexual minorities)?

What does the CTN Offer Social Workers? 5. Sufficient benefit to warrant cost of training and implementing? 6. What is required to train community providers to deliver EBPs? 7. Characteristics of treatment sites that deliver EBPs successfully? 8. Sustainability of practices

1. EBPs for SUD

2. EBPs for Adolescents

3. HIV Prevention

4. Evidence-Based for Marginalized Populations?

5. Cost-Benefit

6. Training in EBPs

7. Treatment Sites

8. Sustainability of EBPs

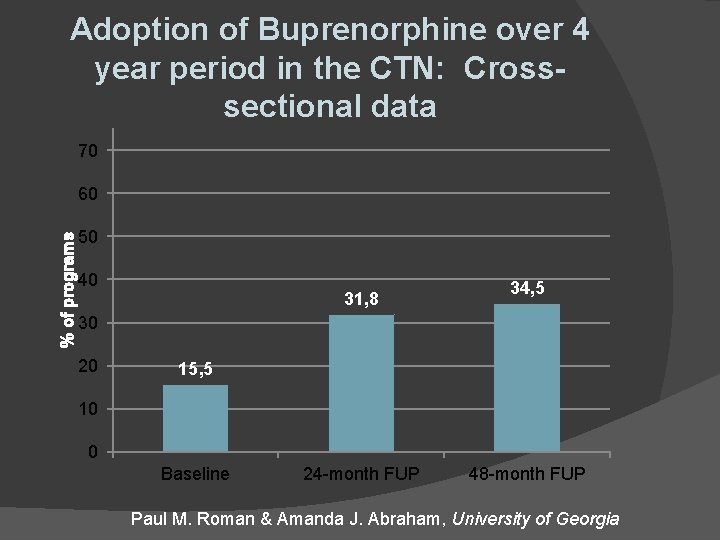
Adoption of Buprenorphine over 4 year period in the CTN: Crosssectional data 70 % of programs 60 50 40 31, 8 34, 5 30 20 15, 5 10 0 Baseline 24 -month FUP 48 -month FUP Paul M. Roman & Amanda J. Abraham, University of Georgia

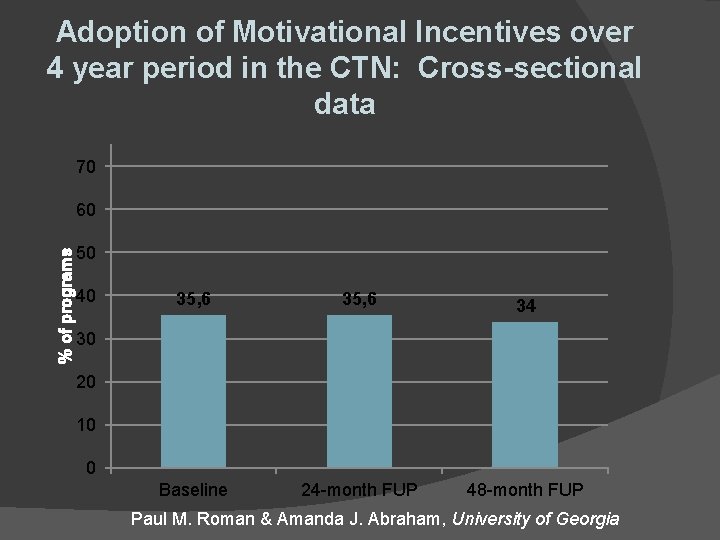
Adoption of Motivational Incentives over 4 year period in the CTN: Cross-sectional data 70 % of programs 60 50 40 35, 6 34 Baseline 24 -month FUP 48 -month FUP 30 20 10 0 Paul M. Roman & Amanda J. Abraham, University of Georgia

http: //ctndisseminationlibrary. org

http: //www. ctndatashare. org/ v Allows researchers to download deidentified data from completed CTN studies to conduct analyses that improve the quality of drug abuse treatment v Data available after primary outcome paper published v Data from 25 CTN studies currently available

The NIDA/SAMHSA Blending Initiative Three components: v Regional Blending Conferences v State Agency Partnerships v Blending Teams

Blending Teams v Use NIDA research findings to design userfriendly science-based tools for use in treatment settings soon after research results are published. v Teams include members from: �SAMHSA-CSAT Addiction Technology Transfer Center (ATTC) Network, �NIDA researchers, and �Community treatment providers participating in the NIDA Drug Abuse Treatment Clinical Trials Network (CTN).

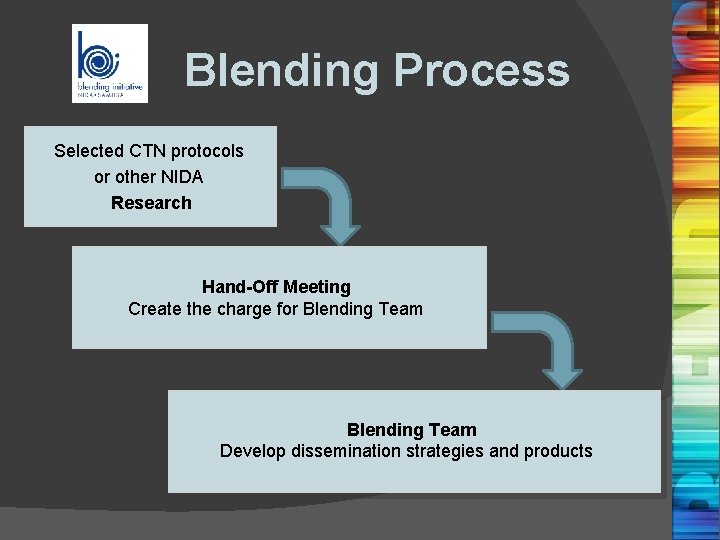
Blending Process Selected CTN protocols or other NIDA Research Hand-Off Meeting Create the charge for Blending Team Develop dissemination strategies and products.

Current Blending Packages

Blending Packages Available in Spanish http: //www. attcnetwork. org/explore/priorityareas/scien ce/blendinginitiative/index. asp

Products Under Development 1. POATS (Prescription Opioid Addiction Treatment Study) 2. Onsite HIV Rapid Testing 3. Buprenorphine for Young Adults (Spanish version)