NICKEL LATERITES characteristics classification and processing options Charles











- Slides: 32

NICKEL LATERITES characteristics, classification and processing options Charles Butt August 2007

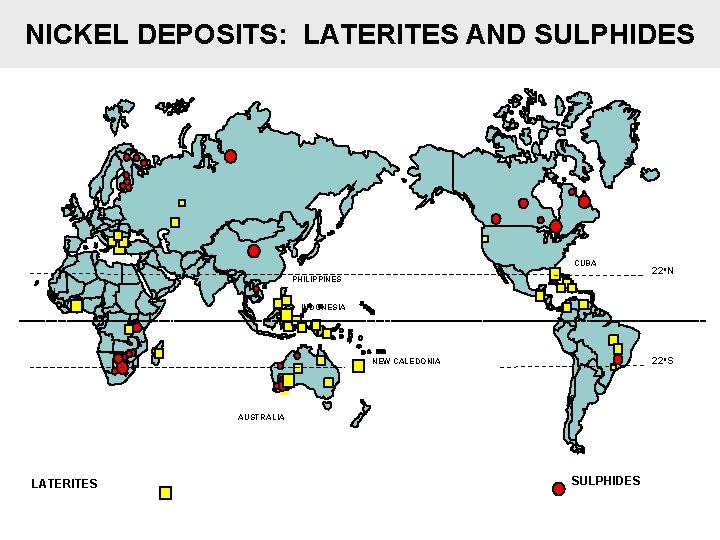
NICKEL DEPOSITS: LATERITES AND SULPHIDES CUBA PHILIPPINES 22 o. N INDONESIA 22 o. S NEW CALEDONIA AUSTRALIA LATERITES SULPHIDES

NICKEL LATERITE • Regolith, derived from ultramafic rocks, that contains commercially exploitable reserves of nickel (and, commonly, cobalt) i. e. , an economic term, implying high grades and/or tonnages of Ni-rich material • ultramafic rocks, >~2500 ppm Ni Peridotite: 40 -90% olivine + pyroxene Dunite: >90% olivine ophiolite, komatiite; layered intrusives (all ± serpentinized)

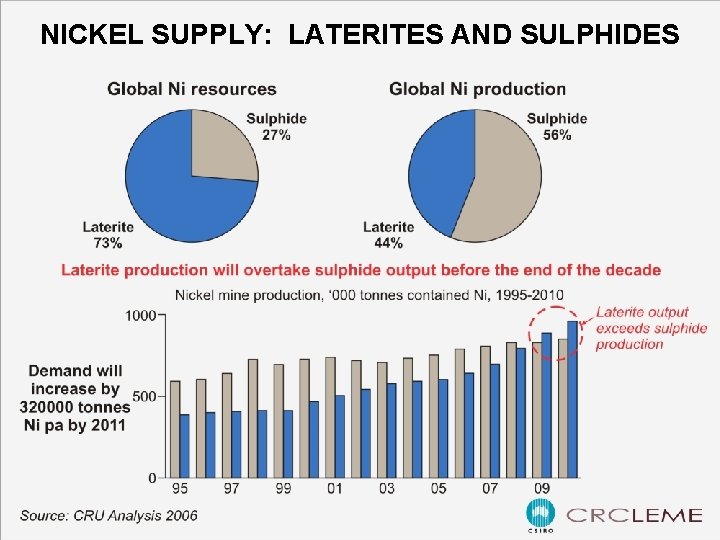
NICKEL SUPPLY: LATERITES AND SULPHIDES

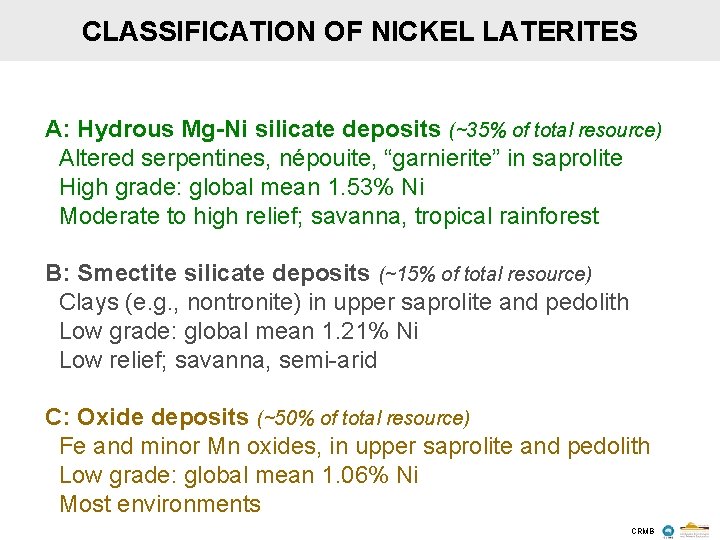
CLASSIFICATION OF NICKEL LATERITES A: Hydrous Mg-Ni silicate deposits (~35% of total resource) Altered serpentines, népouite, “garnierite” in saprolite High grade: global mean 1. 53% Ni Moderate to high relief; savanna, tropical rainforest B: Smectite silicate deposits (~15% of total resource) Clays (e. g. , nontronite) in upper saprolite and pedolith Low grade: global mean 1. 21% Ni Low relief; savanna, semi-arid C: Oxide deposits (~50% of total resource) Fe and minor Mn oxides, in upper saprolite and pedolith Low grade: global mean 1. 06% Ni Most environments CRMB

EAST PINARES Cuba Photo: Mick Elias Oxide

GORO New Caledonia Oxide; some hydrous silicate CRMB

CAWSE Western Australia Oxide CRMB

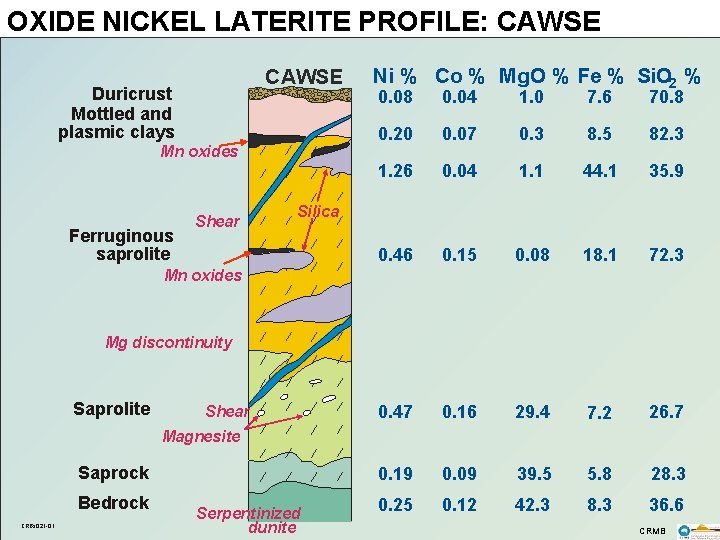
OXIDE NICKEL LATERITE PROFILE: CAWSE Duricrust Mottled and plasmic clays Mn oxides Ferruginous saprolite Shear Ni % Co % Mg. O % Fe % Si. O 2 % 0. 08 0. 04 1. 0 7. 6 70. 8 0. 20 0. 07 0. 3 8. 5 82. 3 1. 26 0. 04 1. 1 44. 1 35. 9 0. 46 0. 15 0. 08 18. 1 72. 3 0. 47 0. 16 29. 4 7. 2 26. 7 0. 19 0. 09 39. 5 5. 8 28. 3 0. 25 0. 12 42. 3 8. 3 36. 6 Silica Mn oxides Mg discontinuity Saprolite Shear Magnesite Saprock Bedrock CRBs 021 -01 Serpentinized dunite CRMB

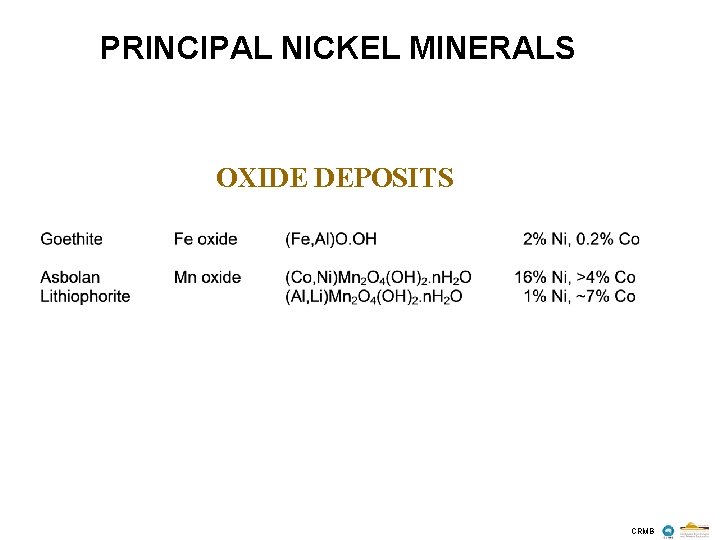
PRINCIPAL NICKEL MINERALS OXIDE DEPOSITS CRMB

PLATEAU New Caledonia Hydrous silicate; minor oxide CRMB

PLATEAU New Caledonia Hydrous silicate CRMB

CIRCE New Caledonia Hydrous silicate “garnierite” ore CRMB

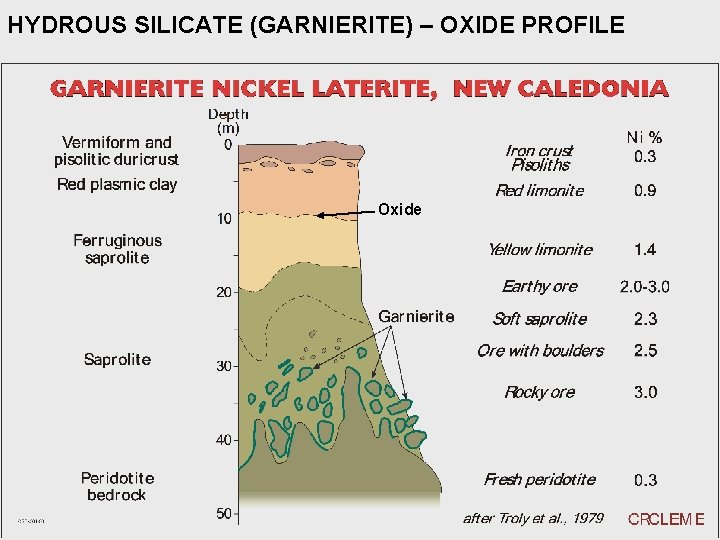
HYDROUS SILICATE (GARNIERITE) – OXIDE PROFILE Oxide

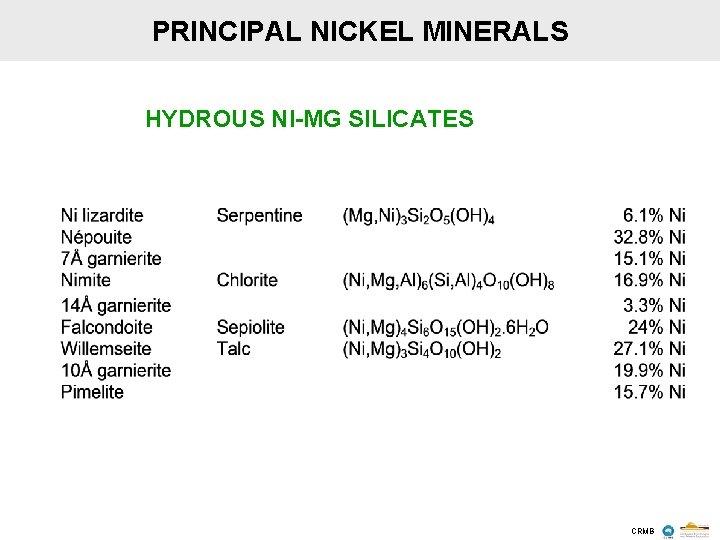
PRINCIPAL NICKEL MINERALS HYDROUS NI-MG SILICATES CRMB

BULONG Western Australia Smectite silicate CRMB

MURRIN Western Australia Smectite silicate CRMB

MURRIN Western Australia Smectite silicate magnesite Photo: Martin Wells

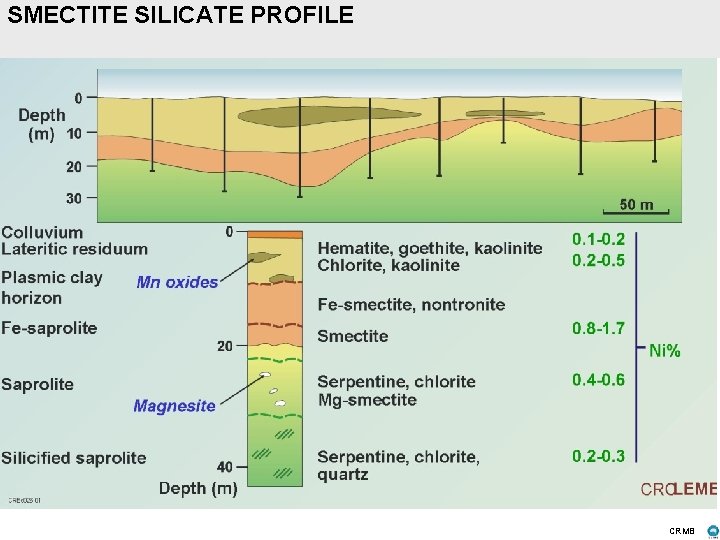
SMECTITE SILICATE PROFILE CRMB

PRINCIPAL NICKEL MINERALS SMECTITE DEPOSITS Minor goethite, asbolan CRMB

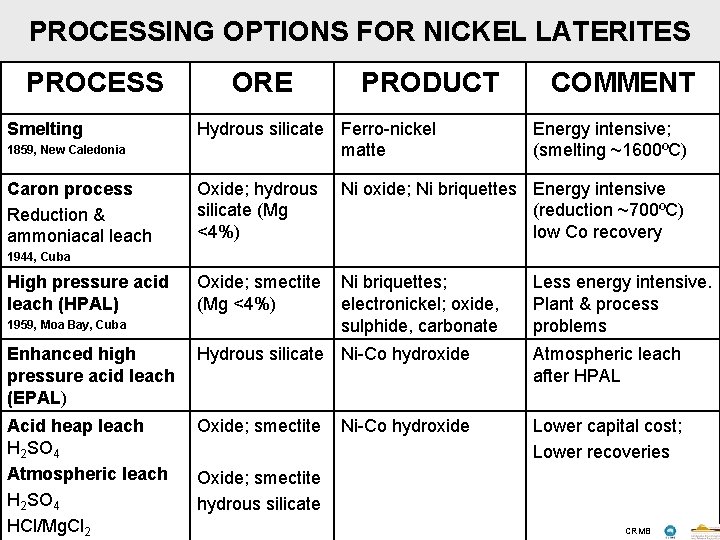
PROCESSING OPTIONS FOR NICKEL LATERITES PROCESS Smelting ORE PRODUCT COMMENT 1859, New Caledonia Hydrous silicate Ferro-nickel matte Energy intensive; (smelting ~1600ºC) Caron process Reduction & ammoniacal leach Oxide; hydrous silicate (Mg <4%) Ni oxide; Ni briquettes Energy intensive (reduction ~700ºC) low Co recovery Oxide; smectite (Mg <4%) Ni briquettes; electronickel; oxide, sulphide, carbonate 1944, Cuba High pressure acid leach (HPAL) 1959, Moa Bay, Cuba Less energy intensive. Plant & process problems Enhanced high pressure acid leach (EPAL) Hydrous silicate Ni-Co hydroxide Atmospheric leach after HPAL Acid heap leach H 2 SO 4 Atmospheric leach H 2 SO 4 HCl/Mg. Cl 2 Oxide; smectite Lower capital cost; Lower recoveries Ni-Co hydroxide Oxide; smectite hydrous silicate CRMB

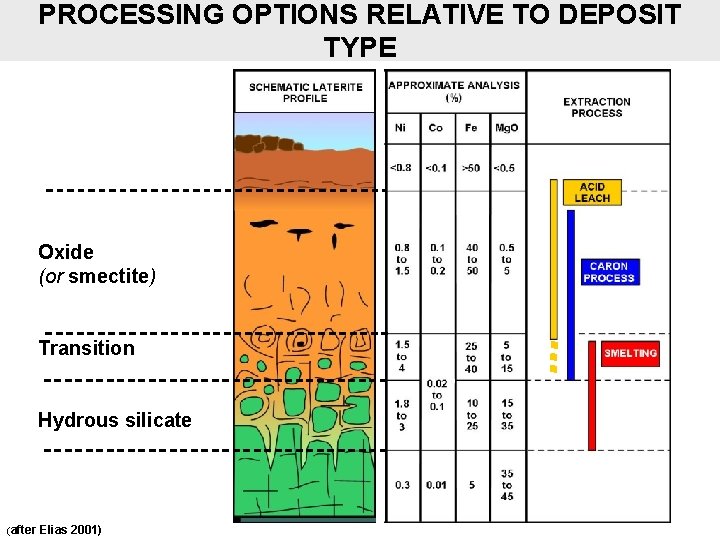
PROCESSING OPTIONS RELATIVE TO DEPOSIT TYPE Oxide (or smectite) Transition Hydrous silicate (after Elias 2001)

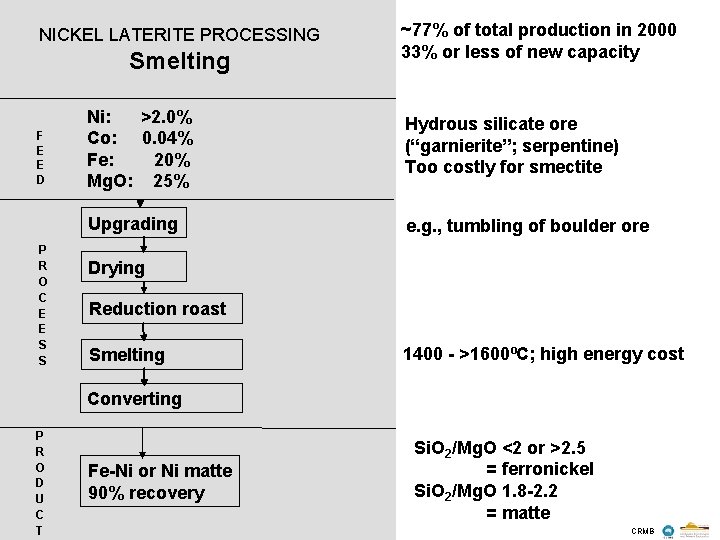
NICKEL LATERITE PROCESSING Smelting F E E D P R O C E E S S ~77% of total production in 2000 33% or less of new capacity Ni: >2. 0% Co: 0. 04% Fe: 20% Mg. O: 25% Hydrous silicate ore (“garnierite”; serpentine) Too costly for smectite Upgrading e. g. , tumbling of boulder ore Drying Reduction roast Smelting 1400 - >1600ºC; high energy cost Converting P R O D U C T Fe-Ni or Ni matte 90% recovery Si. O 2/Mg. O <2 or >2. 5 = ferronickel Si. O 2/Mg. O 1. 8 -2. 2 = matte CRMB

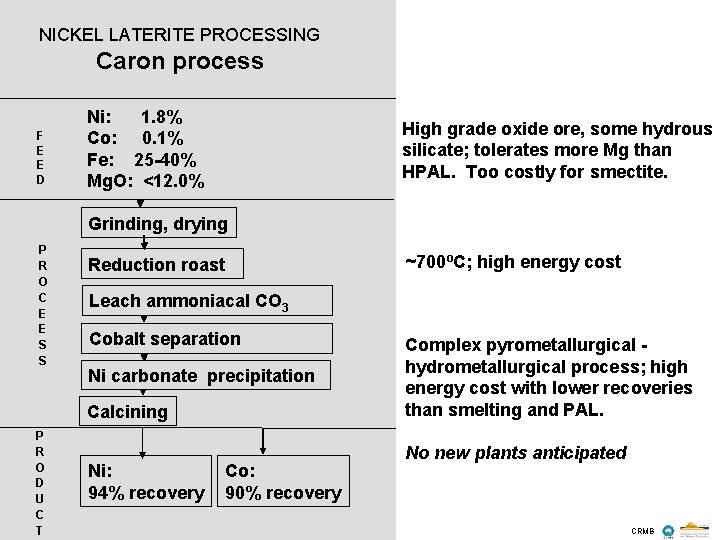
NICKEL LATERITE PROCESSING Caron process F E E D Ni: 1. 8% Co: 0. 1% Fe: 25 -40% Mg. O: <12. 0% High grade oxide ore, some hydrous silicate; tolerates more Mg than HPAL. Too costly for smectite. Grinding, drying P R O C E E S S Reduction roast Leach ammoniacal CO 3 Cobalt separation Ni carbonate precipitation Calcining P R O D U C T ~700ºC; high energy cost Ni: 94% recovery Co: 90% recovery Complex pyrometallurgical hydrometallurgical process; high energy cost with lower recoveries than smelting and PAL. No new plants anticipated CRMB

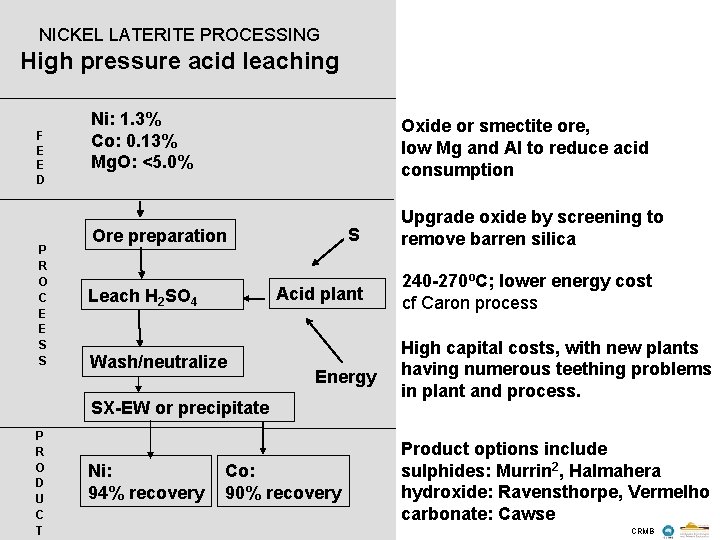
NICKEL LATERITE PROCESSING High pressure acid leaching F E E D P R O C E E S S Ni: 1. 3% Co: 0. 13% Mg. O: <5. 0% Oxide or smectite ore, low Mg and Al to reduce acid consumption Ore preparation Upgrade oxide by screening to remove barren silica S Acid plant Leach H 2 SO 4 Wash/neutralize Energy SX-EW or precipitate P R O D U C T Ni: 94% recovery Co: 90% recovery 240 -270ºC; lower energy cost cf Caron process High capital costs, with new plants having numerous teething problems in plant and process. Product options include sulphides: Murrin 2, Halmahera hydroxide: Ravensthorpe, Vermelho carbonate: Cawse CRMB

Murrin

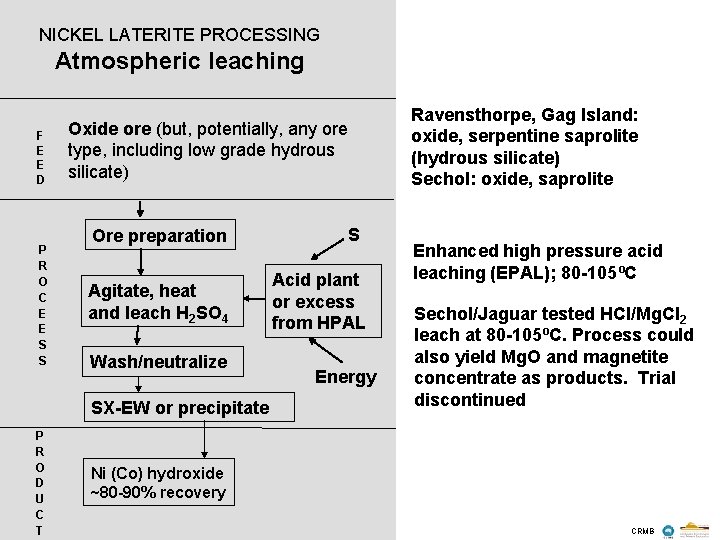
NICKEL LATERITE PROCESSING Atmospheric leaching F E E D P R O C E E S S Oxide ore (but, potentially, any ore type, including low grade hydrous silicate) Ore preparation Agitate, heat and leach H 2 SO 4 Wash/neutralize SX-EW or precipitate P R O D U C T S Acid plant or excess from HPAL Energy Ravensthorpe, Gag Island: oxide, serpentine saprolite (hydrous silicate) Sechol: oxide, saprolite Enhanced high pressure acid leaching (EPAL); 80 -105ºC Sechol/Jaguar tested HCl/Mg. Cl 2 leach at 80 -105ºC. Process could also yield Mg. O and magnetite concentrate as products. Trial discontinued Ni (Co) hydroxide ~80 -90% recovery CRMB

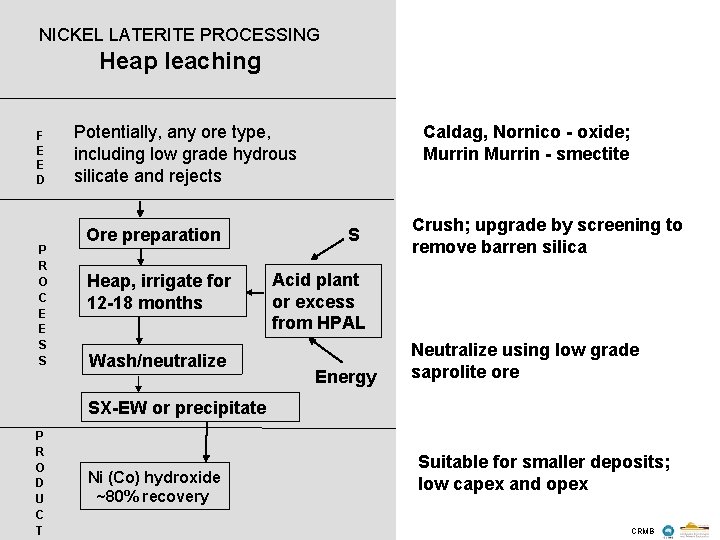
NICKEL LATERITE PROCESSING Heap leaching F E E D P R O C E E S S Caldag, Nornico - oxide; Murrin - smectite Potentially, any ore type, including low grade hydrous silicate and rejects Ore preparation Heap, irrigate for 12 -18 months Wash/neutralize S Crush; upgrade by screening to remove barren silica Acid plant or excess from HPAL Energy Neutralize using low grade saprolite ore SX-EW or precipitate P R O D U C T Ni (Co) hydroxide ~80% recovery Suitable for smaller deposits; low capex and opex CRMB

Çaldağ Heap Leach project, Turkey * Istanbul Çaldağ Izmir * 20 km 50 km Demonstration precipitation plant European Nickel plc 2006 200 m From top of Heap 2 looking at Çaldağ mountain

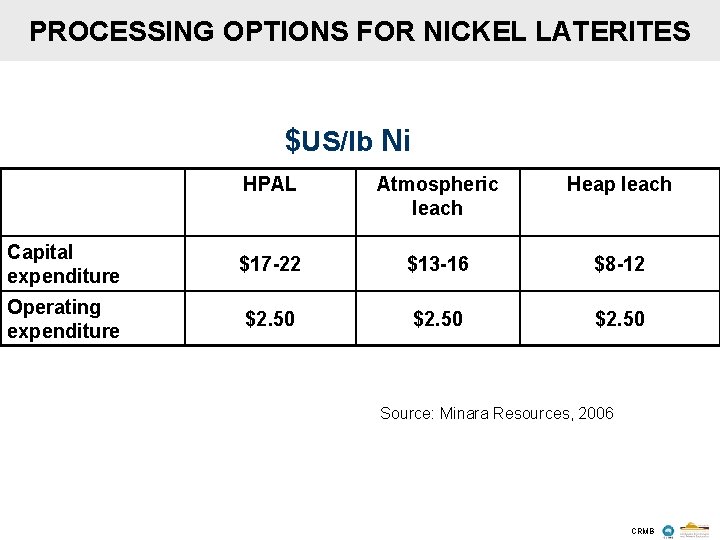
PROCESSING OPTIONS FOR NICKEL LATERITES $US/lb Ni HPAL Atmospheric leach Heap leach Capital expenditure $17 -22 $13 -16 $8 -12 Operating expenditure $2. 50 Source: Minara Resources, 2006 CRMB

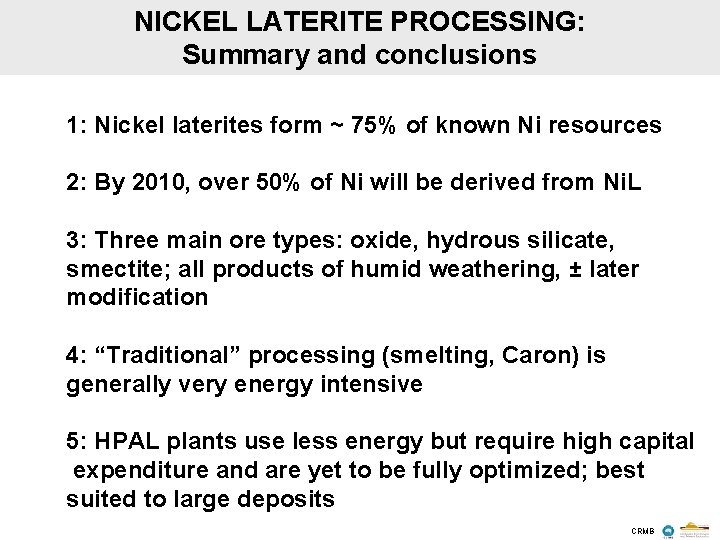
NICKEL LATERITE PROCESSING: Summary and conclusions 1: Nickel laterites form ~ 75% of known Ni resources 2: By 2010, over 50% of Ni will be derived from Ni. L 3: Three main ore types: oxide, hydrous silicate, smectite; all products of humid weathering, ± later modification 4: “Traditional” processing (smelting, Caron) is generally very energy intensive 5: HPAL plants use less energy but require high capital expenditure and are yet to be fully optimized; best suited to large deposits CRMB

NICKEL LATERITE PROCESSING: Summary and conclusions (continued): 6: Acid leaching at lower temperatures and ambient pressures offer lower capital expenditure (but lower recovery). Suited for treating lower grade ore and small or remote deposits 7: Better mineralogical characterization is needed to optimize grade control, beneficiation and processing CRMB