Nexavar in Patients with Renal Cell Carcinoma Naomi






























- Slides: 58

Nexavar in Patients with Renal Cell Carcinoma Naomi B. Haas October 4, 2007

Historical Management of Advanced-Stage RCC • Nephrectomy • Metastectomy – Solitary lesions • Cytokine combination • Combined modalities – Adjunctive nephrectomy prior to cytokine therapy – Cytokine therapy followed by nephrectomy • Clinical trials National Comprehensive Care Network. Clinical Practice Guidelines in Oncology: Kidney Cancer: Version 2. 2006. Jenkintown, PA: National Comprehensive Cancer Network; 2006. Figure adapted from Urban BA, Fishman EK. Radiographics. 2000; 20: 197 -212.

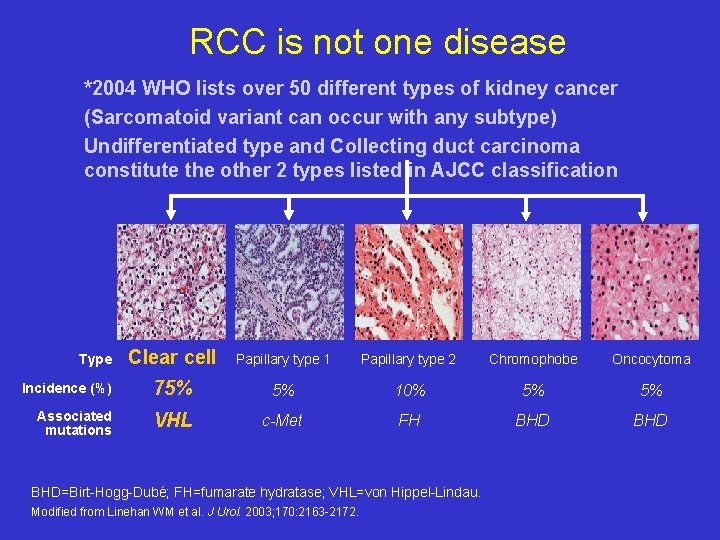
RCC is not one disease *2004 WHO lists over 50 different types of kidney cancer (Sarcomatoid variant can occur with any subtype) Undifferentiated type and Collecting duct carcinoma constitute the other 2 types listed in AJCC classification Type Incidence (%) Associated mutations Clear cell Papillary type 1 Papillary type 2 Chromophobe Oncocytoma 75% 5% 10% 5% 5% VHL c-Met FH BHD BHD=Birt-Hogg-Dubé; FH=fumarate hydratase; VHL=von Hippel-Lindau. Modified from Linehan WM et al. J Urol. 2003; 170: 2163 -2172.


Treatment of Advanced Disease • Based in part on risk factors

MSKCC Risk Factor Model in m. RCC 0 risk factors (n=80 patients) 1 or 2 risk factors (n=269 patients) 3, 4, or 5 risk factors (n=88 patients) 1. 0 Proportion Surviving 0. 9 0. 8 Risk factors associated with worse prognosis 0. 7 • KPS <80 0. 6 • Low serum hemoglobin (13 g/d. L/11. 5 g/d. L: M/F) 0. 5 • High corrected calcium (10 mg/d. L) 0. 4 • High LDH (300 U/L) 0. 3 • Time from Dx to IFN- <1 yr 0. 2 0. 1 0 0 6 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Time From Start of IFN- (years) Motzer RJ et al. J Clin Oncol. 2002; 20: 289 -296.

ADVERSE PROGNOSTIC FACTORS FOR RENAL CELL CARCINOMA Motzer et al, JCO 17: 2530 -2540, 1999 Risk # Risk Factors • Favorable 0 • Intermediate 1 -2 • Poor 3 and + Median Survival 29 mo. 14 mo.

Do patients with advanced disease do better with nephrectomy? Performance status matters This issue has not been addressed in the era of targeted therapy

Advanced Disease Therapy in 2007 • Multitargeted tyrosine kinase inhibitors • Mammalian target of rapamycin (m. Tor) inhibitors • Anti VEGF antibodies • VEGF Trap • Other angiogenesis inhibitorsthrombospondin inhibitors, pure PDGFR and VEGFR inhibitors


The molecular profiles associated with the various histologic RCC subtypes have identified logical targets • Pathways associated with EGFR, AKT/m. TOR, MAPK/MEK, and VEGF are important in RCC • Drugs available in 2007 – Multitargeted TK inhibitors: • sunitinib, sorafenib, (FDA approved) • GW 786034, AG 013736, ABT 869 – Antibodies: • Bevacizumab – Imids: CC-5013 – m. Tor inhibitors: temsirilimus, RAD 001, rapamycin


Common Treatment related side effects

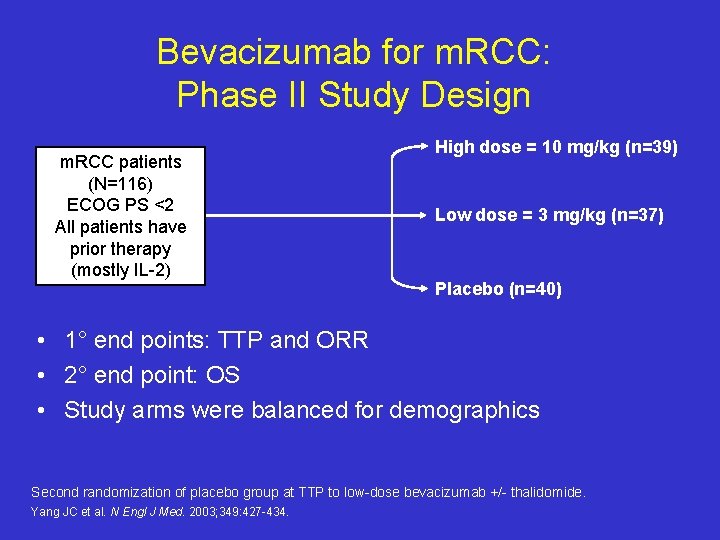
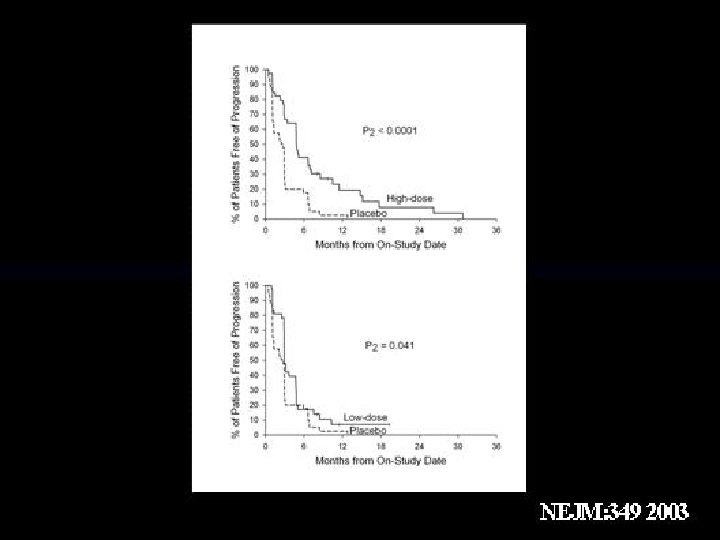
Bevacizumab for m. RCC: Phase II Study Design m. RCC patients (N=116) ECOG PS <2 All patients have prior therapy (mostly IL-2) High dose = 10 mg/kg (n=39) Low dose = 3 mg/kg (n=37) Placebo (n=40) • 1° end points: TTP and ORR • 2° end point: OS • Study arms were balanced for demographics Second randomization of placebo group at TTP to low-dose bevacizumab +/- thalidomide. Yang JC et al. N Engl J Med. 2003; 349: 427 -434.


Bevacizumab for m. RCC: Summary • No significant difference in OS between treatment groups • High dose (10 mg/kg) – PR = 10% (95 CI, 2. 9%– 24. 2%) – Significantly prolonged PFS (median 4. 8 months, P<. 001) – Moderate toxicity profile – No Grade 4 AE or deaths related to therapy • Proteinuria 64% (any grade) • Hypertension 36% (any grade) • Low dose (3 mg/kg) – Not significant

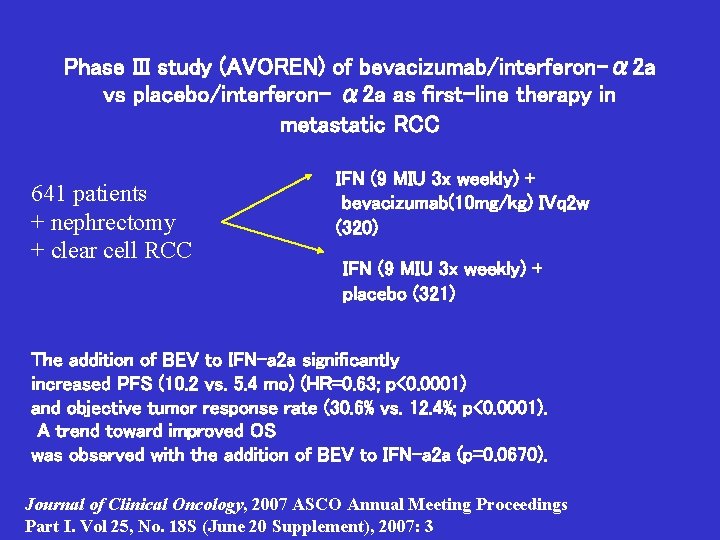
Phase III study (AVOREN) of bevacizumab/interferon-α 2 a vs placebo/interferon- α 2 a as first-line therapy in metastatic RCC 641 patients + nephrectomy + clear cell RCC IFN (9 MIU 3 x weekly) + bevacizumab(10 mg/kg) IVq 2 w (320) IFN (9 MIU 3 x weekly) + placebo (321) The addition of BEV to IFN-a 2 a significantly increased PFS (10. 2 vs. 5. 4 mo) (HR=0. 63; p<0. 0001) and objective tumor response rate (30. 6% vs. 12. 4%; p<0. 0001). A trend toward improved OS was observed with the addition of BEV to IFN-a 2 a (p=0. 0670). Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18 S (June 20 Supplement), 2007: 3

Sorafenib ® (Nexavar ) • Small-molecule receptor TKI 1 • Inhibits VEGFR-2, VEGFR-3, FLT-3, PDGFR, c-KIT, Raf kinases 1 • Formulation: 200 mg tablets 2 • Dosing: 2 tablets bid continuous (1 hr ac or 2 hrs pc)2 • FDA approved December 20, 2005 for advanced RCC 3 CI CF 3 O O N H NH N CH 3 1. Wilhelm SM et al. Cancer Res. 2004; 10: 7099 -7109. 2. Nexavar [package insert]. West Haven, CT: Bayer Pharmaceutical Corporation and Emeryville, CA: Onyx Pharmaceuticals, Inc. ; 2005. 3. Food and Drug Administration. FDA approves new treatment for advanced kidney cancer. Available at: www. fda. gov/bbs/topics/NEWS/2005/NEW 01282. html. Accessed January 24, 2006.

Sorafenib for m. RCC: Phase II (RDT) Progression-Free Survival Proportion of Patients Progression-Free 1. 00 Sorafenib (n=33) Placebo (n=32) Censored 0. 75 Median PFS from randomization Sorafenib=24 weeks Placebo=6 weeks 0. 50 P=. 0087 0. 25 0 84 12 -week period 0 100 200 300 400 Time From Randomization (days) Ratain MJ et al. Presented at: ASCO; May 13 -17, 2005; Orlando FL. 500


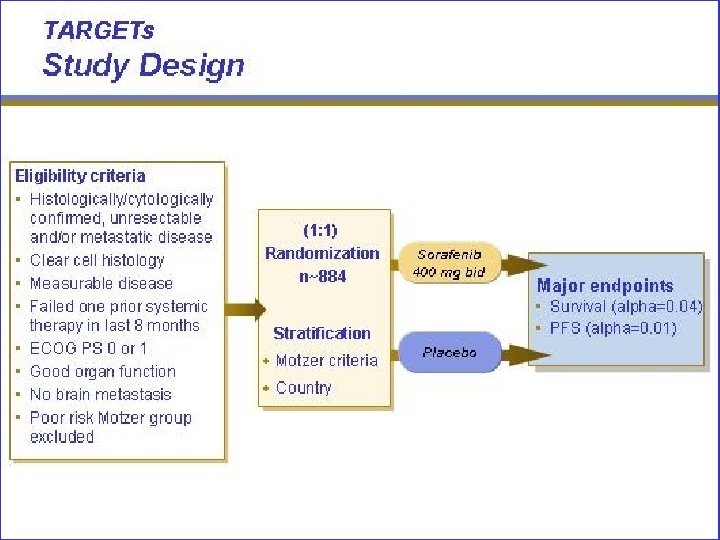
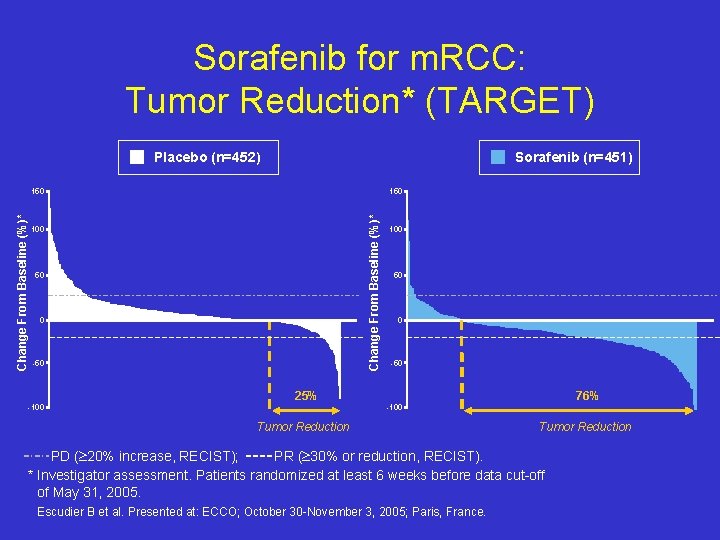
Sorafenib for m. RCC: Tumor Reduction* (TARGET) Placebo (n=452) Sorafenib (n=451) 150 Change From Baseline (%)* 150 100 50 0 -50 25% -100 76% -100 Tumor Reduction PD ( 20% increase, RECIST); PR ( 30% or reduction, RECIST). * Investigator assessment. Patients randomized at least 6 weeks before data cut-off of May 31, 2005. Escudier B et al. Presented at: ECCO; October 30 -November 3, 2005; Paris, France.

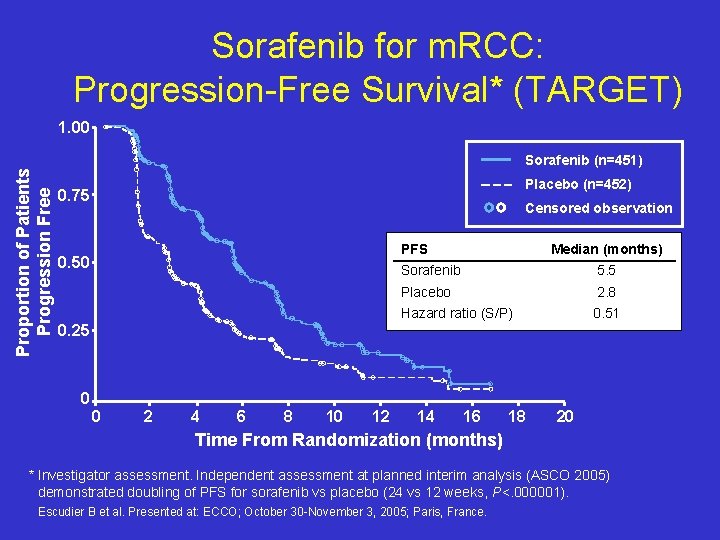
Sorafenib for m. RCC: Progression-Free Survival* (TARGET) 1. 00 Proportion of Patients Progression Free Sorafenib (n=451) Placebo (n=452) 0. 75 Censored observation PFS 0. 50 0. 25 Median (months) Sorafenib 5. 5 Placebo 2. 8 Hazard ratio (S/P) 0. 51 0 0 2 4 6 8 10 12 14 16 18 20 Time From Randomization (months) * Investigator assessment. Independent assessment at planned interim analysis (ASCO 2005) demonstrated doubling of PFS for sorafenib vs placebo (24 vs 12 weeks, P<. 000001). Escudier B et al. Presented at: ECCO; October 30 -November 3, 2005; Paris, France.

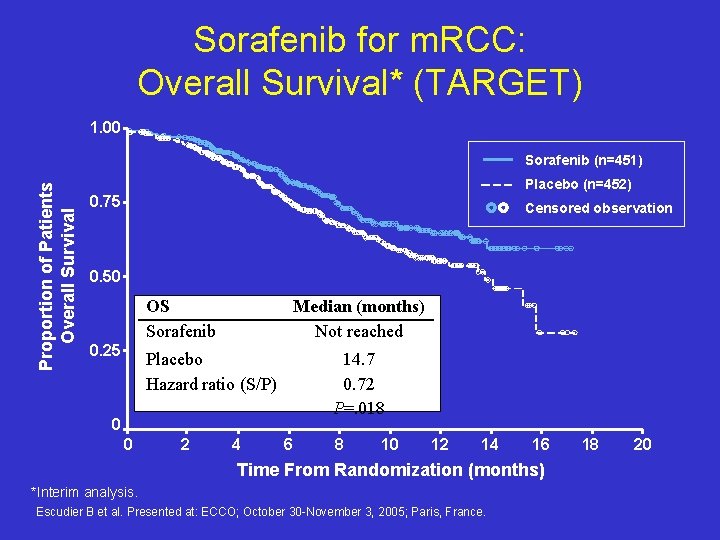
Sorafenib for m. RCC: Overall Survival* (TARGET) 1. 00 Proportion of Patients Overall Survival Sorafenib (n=451) Placebo (n=452) 0. 75 Censored observation 0. 50 OS Sorafenib 0. 25 Median (months) Not reached Placebo Hazard ratio (S/P) 14. 7 0. 72 P=. 018 0 0 2 4 6 8 10 12 14 16 Time From Randomization (months) *Interim analysis. Escudier B et al. Presented at: ECCO; October 30 -November 3, 2005; Paris, France. 18 20

Conclusions • Statistically significant improvement in progression free survival compared to placebo in patients with prior cytokine therapy • Improvement in OS may have been affected by crossover and was not achieved in final analysis

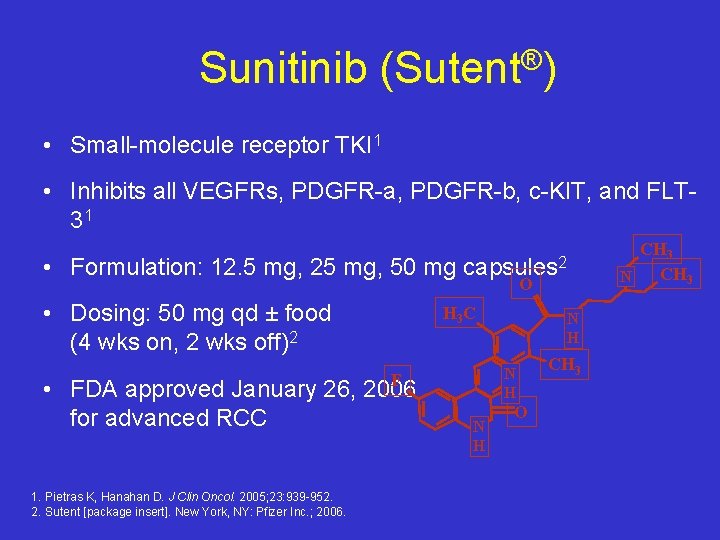
Sunitinib (Sutent®) • Small-molecule receptor TKI 1 • Inhibits all VEGFRs, PDGFR-a, PDGFR-b, c-KIT, and FLT 31 • Formulation: 12. 5 mg, 25 mg, 50 mg • Dosing: 50 mg qd ± food (4 wks on, 2 wks off)2 F • FDA approved January 26, 2006 for advanced RCC 1. Pietras K, Hanahan D. J Clin Oncol. 2005; 23: 939 -952. 2. Sutent [package insert]. New York, NY: Pfizer Inc. ; 2006. capsules 2 O H 3 C N H N H O CH 3 N


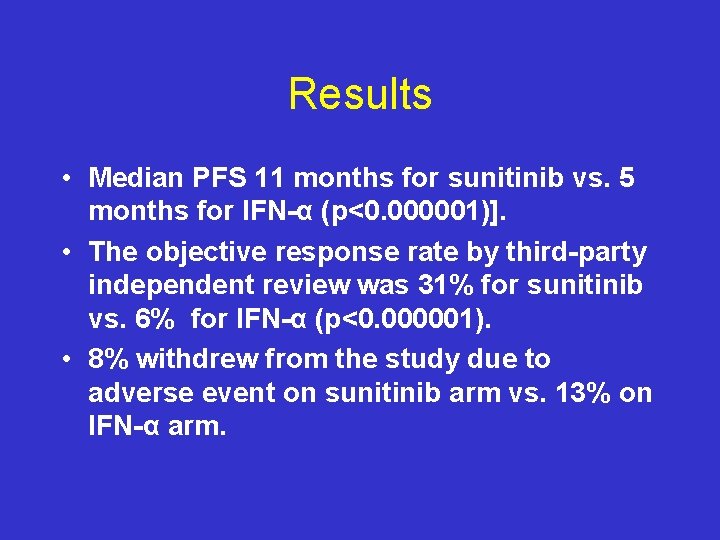
Results • Median PFS 11 months for sunitinib vs. 5 months for IFN-α (p<0. 000001)]. • The objective response rate by third-party independent review was 31% for sunitinib vs. 6% for IFN-α (p<0. 000001). • 8% withdrew from the study due to adverse event on sunitinib arm vs. 13% on IFN-α arm.


Conclusion • Statistically significant improvement in PFS and objective response rate for sunitinib over IFN-α in first-line treatment of patients with metastatic RCC

Targeted Therapy: More Questions Than Answers 1. First Line: Should we combine these agents? 1. Sequentially or concurrently? 2. Vertical inhibition or horizontal inhibition? 2. Which Type of Agent should be used first? m. TKI or m. Tor inhibitor? 3. At Progression Dose escalation of m. TKI vs other mechanism?

6. Treatment duration-are these agents purely angiostatic? 7. Assessment of Response. Which is more important: RECIST or PFS? 8. Predictors of Response Blood flow/ vascularity histology Imaging- PET/CT, DCE/MRI, CT 9. Role of agents-Adjuvant? , First-line? Before or after cytokines? 10. Exposure to agent- drug levels of sunitinib correlated with response 11. Dose escalation of agent –some patients can tolerate dose escalation at the time of progression 12. How to treat non clear cell and other variants

Sequential Use of Nexavar and Sunitinib: Retrospective Analysis in 90 Patients

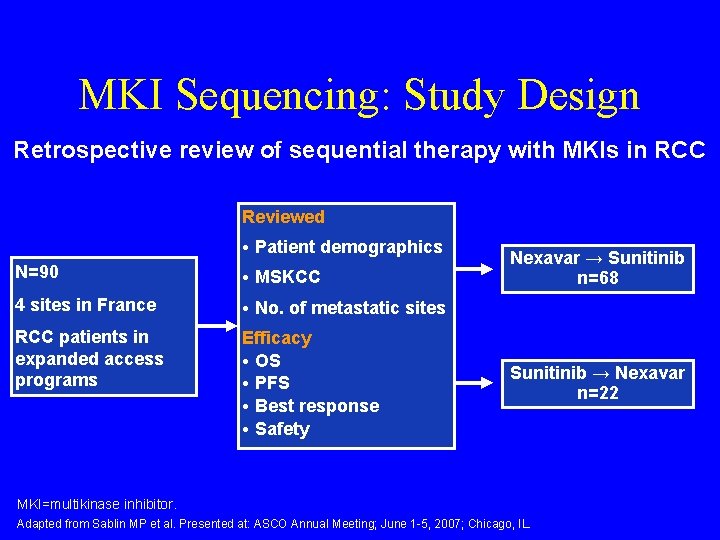
MKI Sequencing: Study Design Retrospective review of sequential therapy with MKIs in RCC Reviewed • Patient demographics N=90 • MSKCC 4 sites in France • No. of metastatic sites RCC patients in expanded access programs Efficacy • OS • PFS • Best response • Safety Nexavar → Sunitinib n=68 Sunitinib → Nexavar n=22 MKI=multikinase inhibitor. Adapted from Sablin MP et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

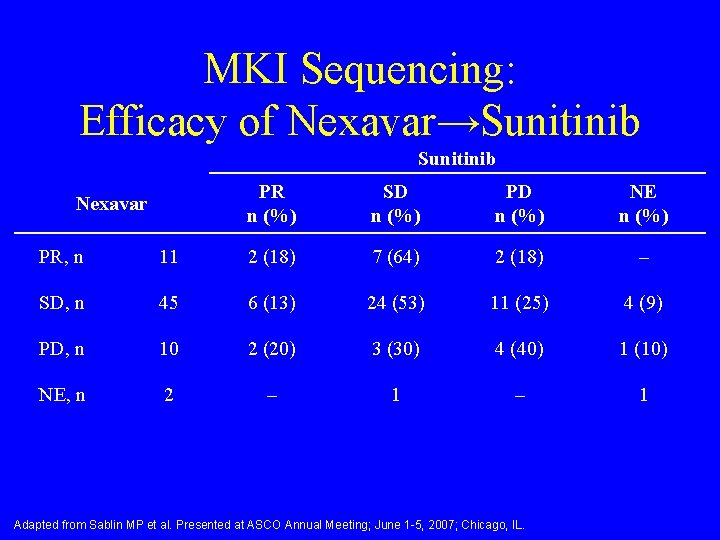
MKI Sequencing: Efficacy of Nexavar→Sunitinib Nexavar PR n (%) SD n (%) PD n (%) NE n (%) PR, n 11 2 (18) 7 (64) 2 (18) – SD, n 45 6 (13) 24 (53) 11 (25) 4 (9) PD, n 10 2 (20) 3 (30) 4 (40) 1 (10) NE, n 2 – 1 Adapted from Sablin MP et al. Presented at ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

MKI Sequencing: Efficacy of Sunitinib→Nexavar Sunitinib PR n (%) SD n (%) PR, n 5 1 (20) 2 (40) SD, n 12 1 (8) 7 (58) 4 (34) PD, n 5 0 3 (60) 2 (40) Adapted from Sablin MP et al. Presented at ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

Phase II Trial of Intrapatient Dose-Escalated-Nexavar in Patients With Metastatic Renal Cell Cancer

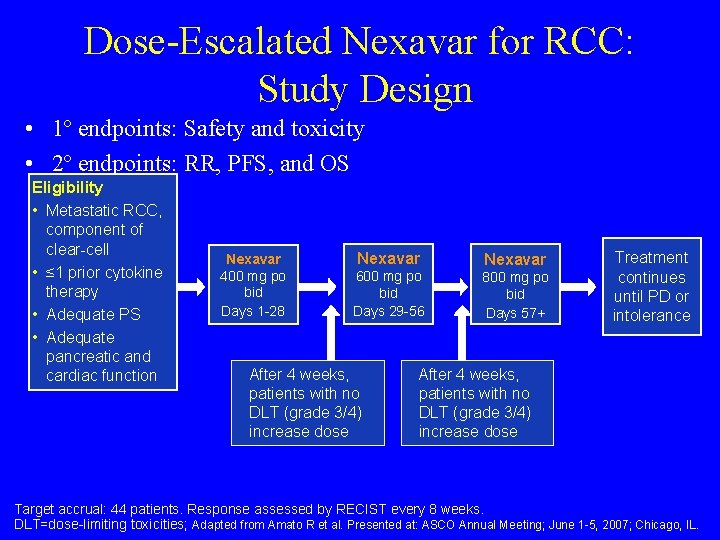
Dose-Escalated Nexavar for RCC: Study Design • 1º endpoints: Safety and toxicity • 2º endpoints: RR, PFS, and OS Eligibility • Metastatic RCC, component of clear-cell • ≤ 1 prior cytokine therapy • Adequate PS • Adequate pancreatic and cardiac function Nexavar 400 mg po bid Days 1 -28 Nexavar 600 mg po bid Days 29 -56 After 4 weeks, patients with no DLT (grade 3/4) increase dose Nexavar 800 mg po bid Days 57+ Treatment continues until PD or intolerance After 4 weeks, patients with no DLT (grade 3/4) increase dose Target accrual: 44 patients. Response assessed by RECIST every 8 weeks. DLT=dose-limiting toxicities; Adapted from Amato R et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

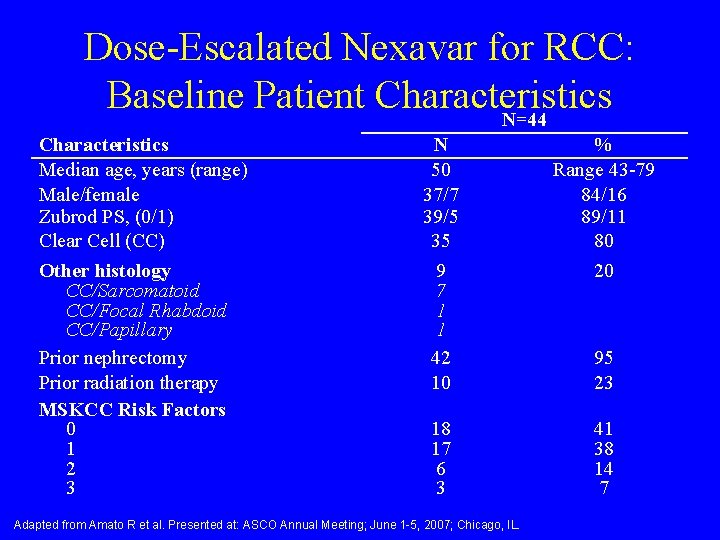
Dose-Escalated Nexavar for RCC: Baseline Patient Characteristics N=44 Characteristics Median age, years (range) Male/female Zubrod PS, (0/1) Clear Cell (CC) Other histology CC/Sarcomatoid CC/Focal Rhabdoid CC/Papillary Prior nephrectomy Prior radiation therapy MSKCC Risk Factors 0 1 2 3 N 50 37/7 39/5 35 9 7 1 1 42 10 % Range 43 -79 84/16 89/11 80 20 18 17 6 3 41 38 14 7 Adapted from Amato R et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL. 95 23

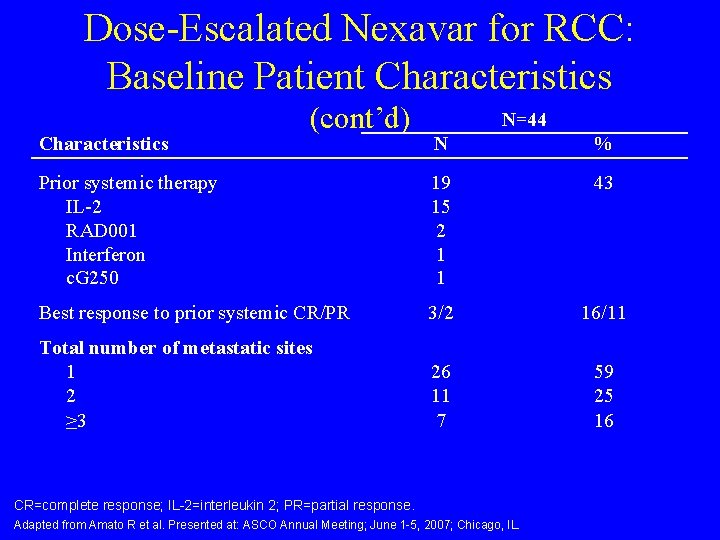
Dose-Escalated Nexavar for RCC: Baseline Patient Characteristics (cont’d) N=44 N % Prior systemic therapy IL-2 RAD 001 Interferon c. G 250 19 15 2 1 1 43 Best response to prior systemic CR/PR 3/2 16/11 Total number of metastatic sites 1 2 ≥ 3 26 11 7 59 25 16 CR=complete response; IL-2=interleukin 2; PR=partial response. Adapted from Amato R et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

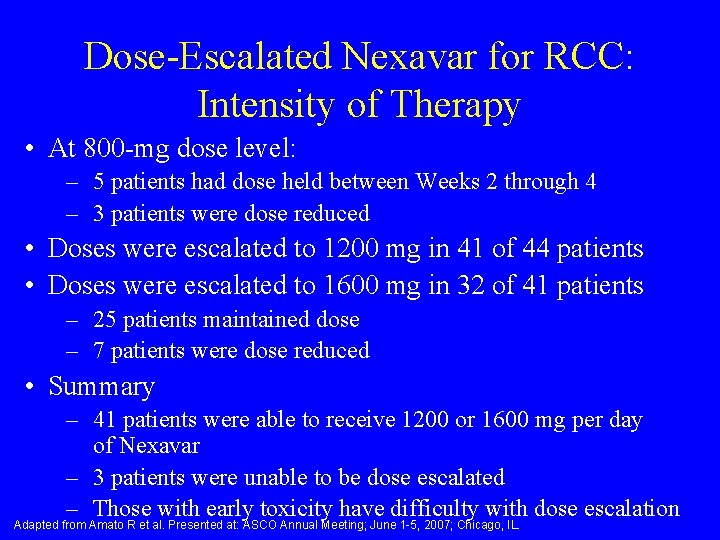
Dose-Escalated Nexavar for RCC: Intensity of Therapy • At 800 -mg dose level: – 5 patients had dose held between Weeks 2 through 4 – 3 patients were dose reduced • Doses were escalated to 1200 mg in 41 of 44 patients • Doses were escalated to 1600 mg in 32 of 41 patients – 25 patients maintained dose – 7 patients were dose reduced • Summary – 41 patients were able to receive 1200 or 1600 mg per day of Nexavar – 3 patients were unable to be dose escalated – Those with early toxicity have difficulty with dose escalation Adapted from Amato R et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

Dose-Escalated Nexavar for RCC: Best Response by RECIST No. of Patients (%) Complete response 7 16 Partial response 17 39 Stable disease ≥ 6 months 9 20 Progression defined as ≤ 4 months 11 25 Best Response Adapted from Amato R et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

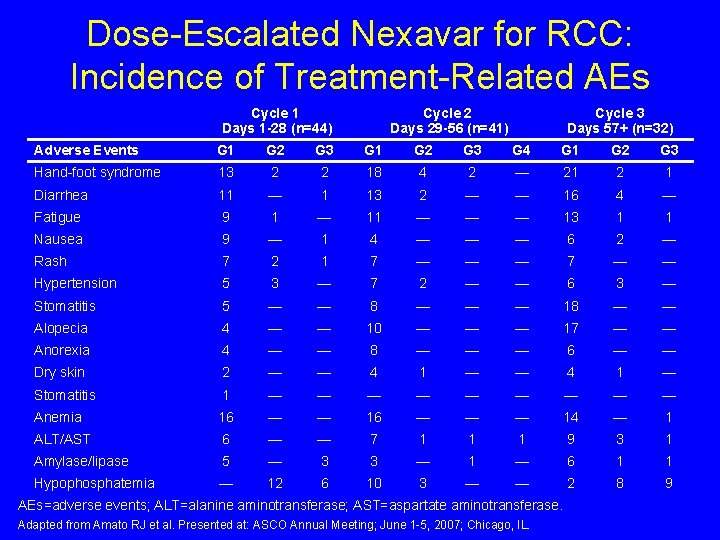
Dose-Escalated Nexavar for RCC: Incidence of Treatment-Related AEs Cycle 1 Days 1 -28 (n=44) Cycle 2 Days 29 -56 (n=41) Cycle 3 Days 57+ (n=32) Adverse Events G 1 G 2 G 3 G 4 G 1 G 2 G 3 Hand-foot syndrome 13 2 2 18 4 2 — 21 2 1 Diarrhea 11 — 1 13 2 — — 16 4 — Fatigue 9 1 — 11 — — — 13 1 1 Nausea 9 — 1 4 — — — 6 2 — Rash 7 2 1 7 — — — 7 — — Hypertension 5 3 — 7 2 — — 6 3 — Stomatitis 5 — — 8 — — — 18 — — Alopecia 4 — — 10 — — — 17 — — Anorexia 4 — — 8 — — — 6 — — Dry skin 2 — — 4 1 — Stomatitis 1 — — — — — Anemia 16 — — — 14 — 1 ALT/AST 6 — — 7 1 1 1 9 3 1 Amylase/lipase 5 — 3 3 — 1 — 6 1 1 Hypophosphatemia — 12 6 10 3 — — 2 8 9 AEs=adverse events; ALT=alanine aminotransferase; AST=aspartate aminotransferase. Adapted from Amato RJ et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

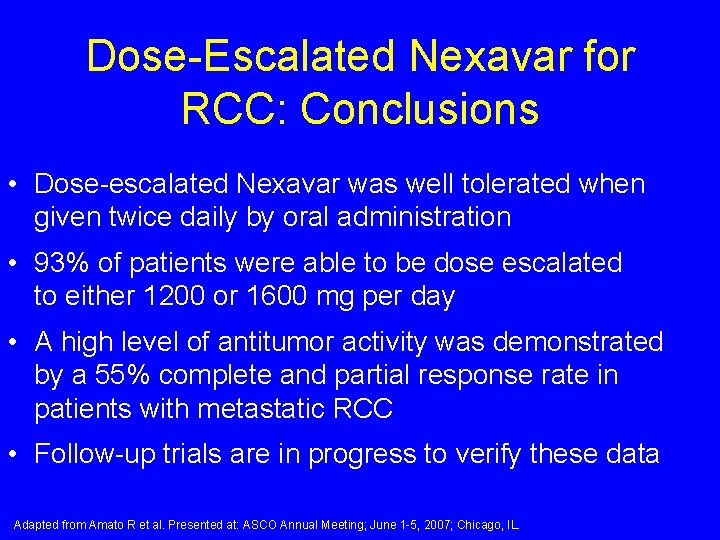
Dose-Escalated Nexavar for RCC: Conclusions • Dose-escalated Nexavar was well tolerated when given twice daily by oral administration • 93% of patients were able to be dose escalated to either 1200 or 1600 mg per day • A high level of antitumor activity was demonstrated by a 55% complete and partial response rate in patients with metastatic RCC • Follow-up trials are in progress to verify these data Adapted from Amato R et al. Presented at: ASCO Annual Meeting; June 1 -5, 2007; Chicago, IL.

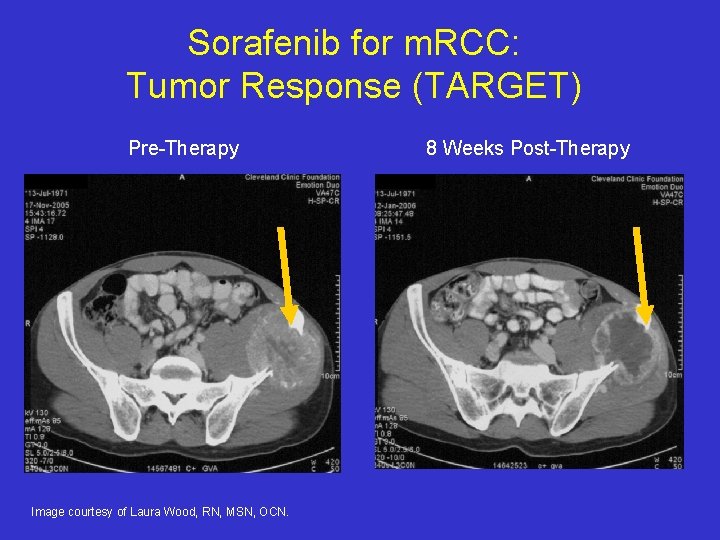
Sorafenib for m. RCC: Tumor Response (TARGET) Pre-Therapy Image courtesy of Laura Wood, RN, MSN, OCN. 8 Weeks Post-Therapy

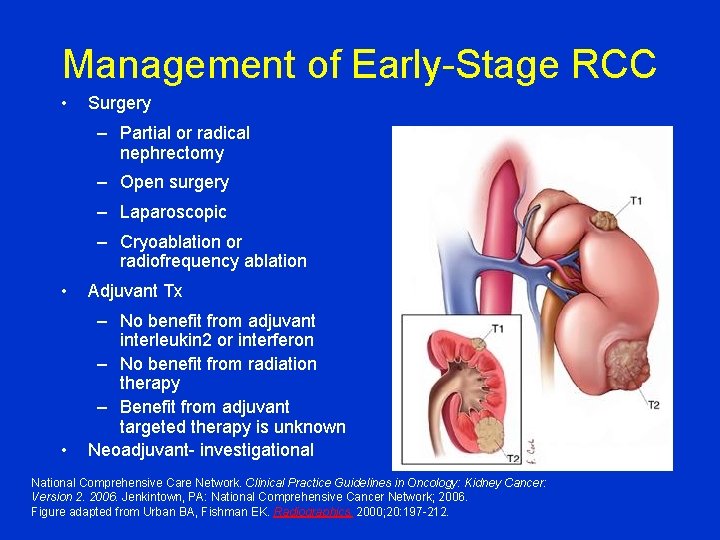
Management of Early-Stage RCC • Surgery – Partial or radical nephrectomy – Open surgery – Laparoscopic – Cryoablation or radiofrequency ablation • Adjuvant Tx • – No benefit from adjuvant interleukin 2 or interferon – No benefit from radiation therapy – Benefit from adjuvant targeted therapy is unknown Neoadjuvant- investigational National Comprehensive Care Network. Clinical Practice Guidelines in Oncology: Kidney Cancer: Version 2. 2006. Jenkintown, PA: National Comprehensive Cancer Network; 2006. Figure adapted from Urban BA, Fishman EK. Radiographics. 2000; 20: 197 -212.

Predictors of Relapse • Prognostic models based on postoperative score: – – UCLA Integrated Staging System (UISS) Leibovich Model Frank Model (SSIGN) Kattan Model • Prognostic Models based on preoperative score: – Yaycioglu – Cindolo

Contributors to Prognosis • • Pathologic stage Histology Fuhrman Grade Nuclear grade Performance status Necrosis Size Clinical Presentation

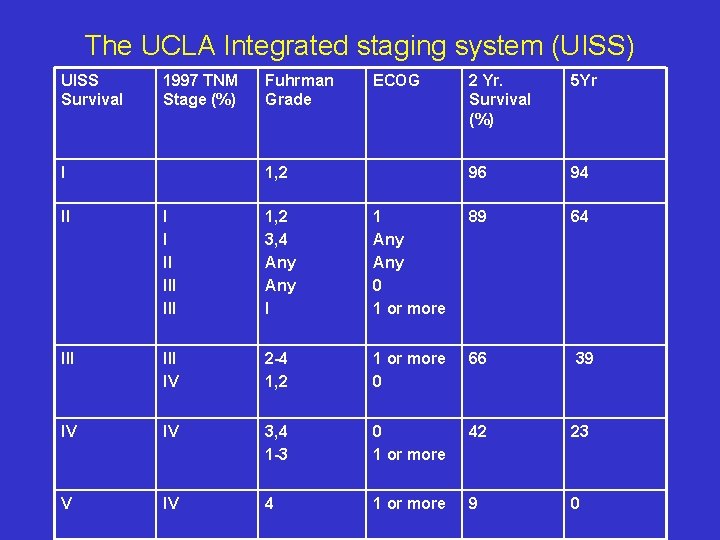
The UCLA Integrated staging system (UISS) UISS Survival 1997 TNM Stage (%) I Fuhrman Grade ECOG 1, 2 2 Yr. Survival (%) 5 Yr 96 94 II I I II III 1, 2 3, 4 Any I 1 Any 0 1 or more 89 64 III IV 2 -4 1, 2 1 or more 0 66 39 IV IV 3, 4 1 -3 0 1 or more 42 23 V IV 4 1 or more 9 0

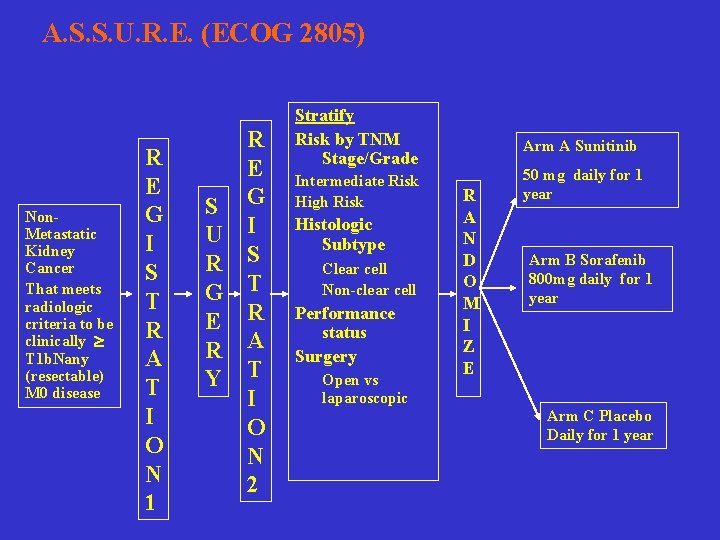
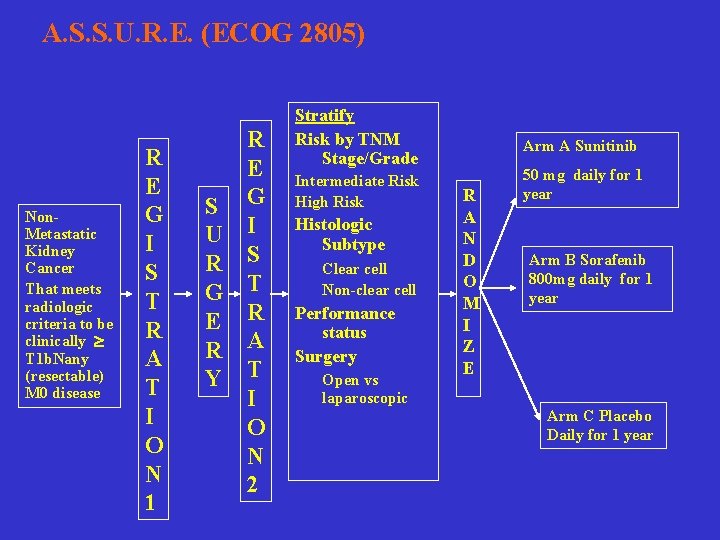
A. S. S. U. R. E. (ECOG 2805) Non. Metastatic Kidney Cancer That meets radiologic criteria to be clinically T 1 b. Nany (resectable) M 0 disease R E G I S T R A T I O N 1 R E S G U I R S G T E R R A Y T I O N 2 Stratify Risk by TNM Stage/Grade Intermediate Risk High Risk Histologic Subtype Clear cell Non-clear cell Performance status Surgery Open vs laparoscopic Arm A Sunitinib R A N D O M I Z E 50 mg daily for 1 year Arm B Sorafenib 800 mg daily for 1 year Arm C Placebo Daily for 1 year

Objectives Primary Question: • Can adjuvant therapy with an oral raf kinase inhibitor/ receptor tyrosine kinase inhibitor (Sorafenib) or pure receptor tyrosine kinase inhibitor (Sunitinib) improve disease-free survival in locally advanced RCC over placebo after surgical resection? • Primary endpoint is DFS

Secondary Objectives • • • Overall survival Toxicity in the adjuvant setting Prospective collection of tumor and biologic specimens /Correlatives: - Measures of angiogenesis - Mutational analyses (oncogenes/TSG) - Hypermethylation - Polymorphisms

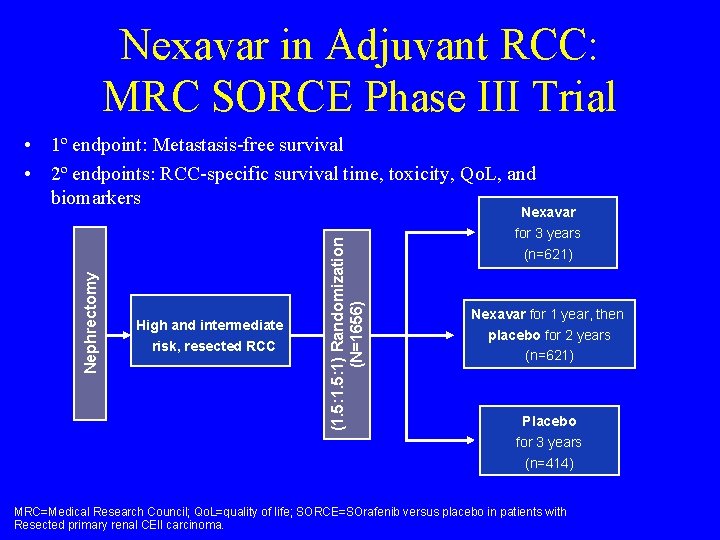
Nexavar in Adjuvant RCC: MRC SORCE Phase III Trial • 1º endpoint: Metastasis-free survival • 2º endpoints: RCC-specific survival time, toxicity, Qo. L, and biomarkers High and intermediate risk, resected RCC (1. 5: 1) Randomization (N=1656) Nephrectomy Nexavar for 3 years (n=621) Nexavar for 1 year, then placebo for 2 years (n=621) Placebo for 3 years (n=414) MRC=Medical Research Council; Qo. L=quality of life; SORCE=SOrafenib versus placebo in patients with Resected primary renal CEll carcinoma.

Developments • ECOG BEST Trial (4 arm) of Doublet Targeted therapy (tem/bev vs sorafenib/bev vs sorafenib/tem • Temsirolimus versus sunitinib trial for non clear cell RCC • m. TKI to m. TOR vs m. Tor to m. TKI trial • Adjuvant trials SORCE and ASSURE are ongoing

Conclusions • Surgery remains the most effective therapy for early RCC and plays a role in advanced RCC • New molecular targets have been identified which are critical to the pathogenesis of different types of RCC and new targeted therapies are replacing traditional biologic therapies • The ultimate role that these agents will play in RCC has yet to be decided

Common Treatment related side effects

Targeted Therapy: More Questions Than Answers 1. First Line: Should we combine these agents? 1. Sequentially or concurrently? 2. Vertical inhibition or horizontal inhibition? 2. Which Type of Agent should be used first? m. TKI or m. Tor inhibitor? 3. At Progression Dose escalation of m. TKI vs other mechanism?

6. Treatment duration-are these agents purely angiostatic? 7. Assessment of Response. Which is more important: RECIST or PFS? 8. Predictors of Response Blood flow/ vascularity histology Imaging- PET/CT, DCE/MRI, CT 9. Role of agents-Adjuvant? , First-line? Before or after cytokines? 10. Exposure to agent- drug levels of sunitinib correlated with response 11. Dose escalation of agent –some patients can tolerate dose escalation at the time of progression 12. How to treat non clear cell and other variants

A. S. S. U. R. E. (ECOG 2805) Non. Metastatic Kidney Cancer That meets radiologic criteria to be clinically T 1 b. Nany (resectable) M 0 disease R E G I S T R A T I O N 1 R E S G U I R S G T E R R A Y T I O N 2 Stratify Risk by TNM Stage/Grade Intermediate Risk High Risk Histologic Subtype Clear cell Non-clear cell Performance status Surgery Open vs laparoscopic Arm A Sunitinib R A N D O M I Z E 50 mg daily for 1 year Arm B Sorafenib 800 mg daily for 1 year Arm C Placebo Daily for 1 year
 Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Naphthylamine
Naphthylamine Nexavar
Nexavar Carcinoma renal de células claras fuhrman
Carcinoma renal de células claras fuhrman Distinguish between renal corpuscle and renal tubule
Distinguish between renal corpuscle and renal tubule Basal cell carcinoma
Basal cell carcinoma Boy or girl hedgehog
Boy or girl hedgehog Squamous cell carcinoma
Squamous cell carcinoma Anaplastic squamous cell carcinoma
Anaplastic squamous cell carcinoma Kode icd 10 basal cell carcinoma
Kode icd 10 basal cell carcinoma Lichen sclerosus vulvare
Lichen sclerosus vulvare Squamous cell carcinoma louisiana
Squamous cell carcinoma louisiana Bcc pathology
Bcc pathology Life expectancy of sickle cell patients
Life expectancy of sickle cell patients Epitheleal
Epitheleal Carcinoma in situ
Carcinoma in situ Cin
Cin Carcinoma squamoso polmone
Carcinoma squamoso polmone -carcinoma
-carcinoma Tumor carcinoide
Tumor carcinoide Follicular carcinoma of thyroid
Follicular carcinoma of thyroid Carcinoma of stomach
Carcinoma of stomach Breast ca tnm staging
Breast ca tnm staging Pheochromocytoma
Pheochromocytoma Emivulvectomia
Emivulvectomia Carcinoma
Carcinoma Thyroid pathology
Thyroid pathology Normal pancreas histology
Normal pancreas histology Neuro derm
Neuro derm Ganglios linfaticos vulva
Ganglios linfaticos vulva Carcinoma on scalp
Carcinoma on scalp Arteria axilar
Arteria axilar Cancer de pulmon
Cancer de pulmon Carcinoma de mama
Carcinoma de mama Carcinoma epidermoide
Carcinoma epidermoide Carcinoma de celulas escamosas
Carcinoma de celulas escamosas Esophagus
Esophagus Endocirne glands
Endocirne glands Brown tumor
Brown tumor Neoplasia
Neoplasia Carcinoma tubular de mama
Carcinoma tubular de mama Carcinoma bronquioloalveolar
Carcinoma bronquioloalveolar Urinary bladder carcinoma
Urinary bladder carcinoma Carcinoma squamoso polmone
Carcinoma squamoso polmone Hnpcc
Hnpcc Carcinoma in situ
Carcinoma in situ Pleomorphic adenomas
Pleomorphic adenomas Hydrohepatosis
Hydrohepatosis Carcinoma comedonico
Carcinoma comedonico Plasmocitoma
Plasmocitoma Breast papillary carcinoma
Breast papillary carcinoma Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features Thymus
Thymus Carcinoma epidermoide microinfiltrante
Carcinoma epidermoide microinfiltrante Dr. aldo azael garza galindo
Dr. aldo azael garza galindo Mucoepidermoid carcinoma pathology outlines
Mucoepidermoid carcinoma pathology outlines Naomi conzo
Naomi conzo Naomi latorraca
Naomi latorraca