New Technologies in cervical cancer screening Cosette Wheeler









- Slides: 16

New Technologies in cervical cancer screening Cosette Wheeler, University of New Mexico Albuquerque, New Mexico

Human Papillomavirus Infections Most common sexually transmitted infections • 20 million prevalent infections • 5. 5 million incident infections per year • Most infections are self-limited and clear in < 1 -2 years, native immunity ?

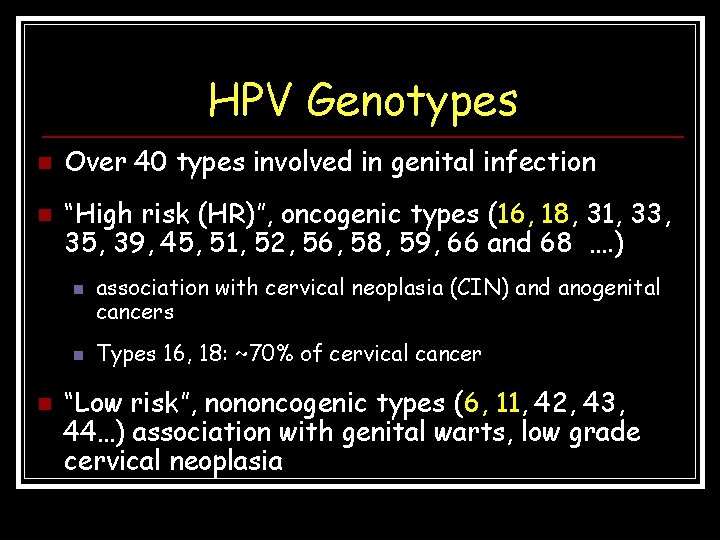
HPV Genotypes n n Over 40 types involved in genital infection “High risk (HR)”, oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68 …. ) n n n association with cervical neoplasia (CIN) and anogenital cancers Types 16, 18: ~70% of cervical cancer “Low risk”, nononcogenic types (6, 11, 42, 43, 44…) association with genital warts, low grade cervical neoplasia

Natural History of HPV Infections and Cervical Cancer: Factors & Co-factors N O R M A L HPV INFECTION • Sexual behavior # • Hygiene LSIL • Immune surveillance & response # • Condom use # • Genetic susceptibility # • Circumcision • Viral factors # INVASIVE CANCER HSIL • Smoking # • Parity # • OC use # • Immunosuppresion • HIV • Other STIs # • HPV genotypes # • Cervical inflammation • Early AFSI # • HPV variants # • High NOSP # • Multiple types # • C. trachomatis • High-risk male partners # • Viral load # • HSV-2 # • Viral integration #

Estimated Annual Abnormal Pap Tests, U. S. CA 15, 000 <15, 000 Ca HSIL 300, 000 HSIL LSIL 1, 250, 000 LSIL 2 -3 X 1, 000 ASC-US Modified from Hildesheim, A. , National Cancer Institute 1, 000 ASC-US 2, 000

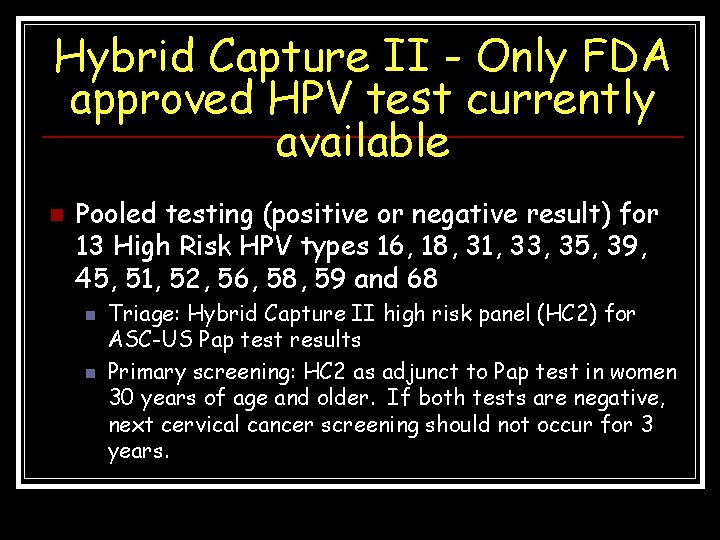
Hybrid Capture II - Only FDA approved HPV test currently available n Pooled testing (positive or negative result) for 13 High Risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 n n Triage: Hybrid Capture II high risk panel (HC 2) for ASC-US Pap test results Primary screening: HC 2 as adjunct to Pap test in women 30 years of age and older. If both tests are negative, next cervical cancer screening should not occur for 3 years.

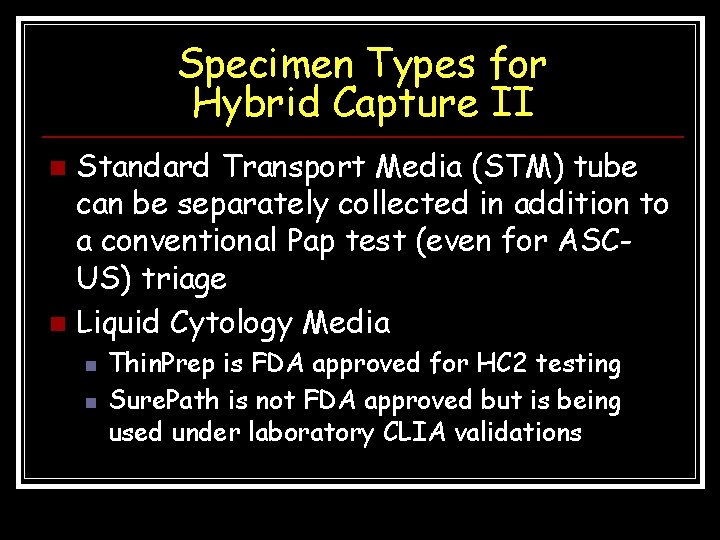
Specimen Types for Hybrid Capture II Standard Transport Media (STM) tube can be separately collected in addition to a conventional Pap test (even for ASCUS) triage n Liquid Cytology Media n n n Thin. Prep is FDA approved for HC 2 testing Sure. Path is not FDA approved but is being used under laboratory CLIA validations

Less than Useful Applications of HPV testing I n There are no recommendations supporting HPV DNA testing as an STI screening test n n Genital HPVs are ubiquitous in young women and presence or absence of HPV does not have prognostic value for disease detection in women 30 years and under Although HPV DNA testing “with” Pap is recommended in women 30 years and older – “throwing in” CT/NG testing because liquid Pap media makes this possible may be inappropriate in this age group

Less than Useful Applications of HPV testing II n n Not recommended in men for any reason Not recommended as part of HPV vaccine decision making n n HR HPV pooled testing is positive or negative for any of 13 HPV genotypes Even if HPV genotype specific HPV 16 and 18 testing becomes available, HPV DNA does not measure past HPV exposure and the probability of detecting all 4 HPV vaccine types is small HPV tests that have clinical utility are not designed to be ultrasensitive Not recommended for individual providers to “monitor” HPV vaccine outcomes (e. g. , in vaccinated women who have subsequent abnormalities) – this is a public health surveillance activity

Several New HPV tests are under evaluation in clinical trials n n n Roche Amplicor - PCR-based assay that detects a pool of 13 high risk HPV types Roche Linear Array - PCR-based assay that detects 37 individual HPV genotypes and uses an internal beta -globin control amplicon Gen. Probe APTIMA HPV RNA test Third Wave Invader HPV test Norchip HPV RNA test Others biomarkers assays including assays that target cell cycle markers (e. g. p 16) and methylation or being evaluated

Future HPV test applications n In women >30, HR HPV testing may replace Pap as our primary cervical screen with reflex to cytology in HPV + n n Algorithms for HPV genotyping utility in women >30 n n n Studies are being conducted to determine management of women who are HPV positive but cytology negative Are HPV 16, 18, 31, 33, 45 of greater risk of disease - can there be more cost-effectively triage of women to colposcopy Will persistent type-specific HPV infections play a role in clinical management - what will be the interval for “persistence” classification Practical considerations make implementation of complex clinical algorithms difficult

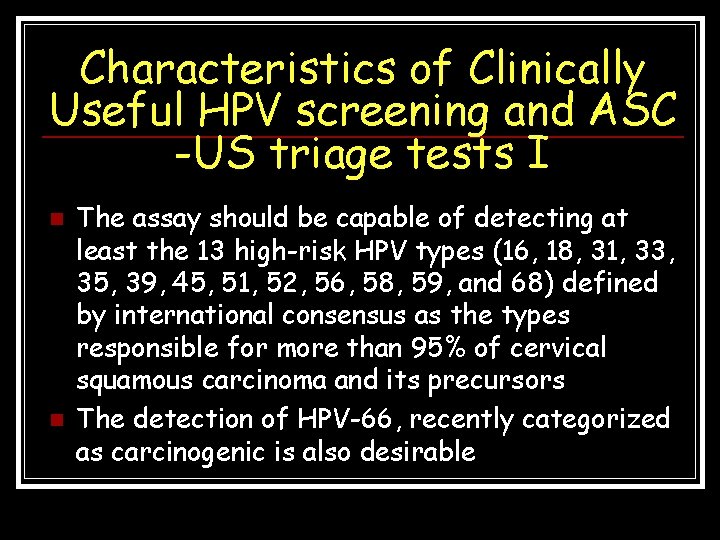
Characteristics of Clinically Useful HPV screening and ASC -US triage tests I n n The assay should be capable of detecting at least the 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) defined by international consensus as the types responsible for more than 95% of cervical squamous carcinoma and its precursors The detection of HPV-66, recently categorized as carcinogenic is also desirable

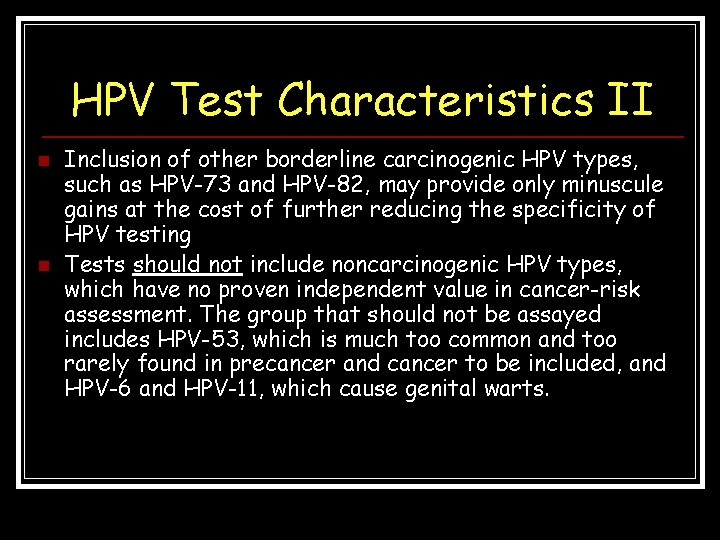
HPV Test Characteristics II n n Inclusion of other borderline carcinogenic HPV types, such as HPV-73 and HPV-82, may provide only minuscule gains at the cost of further reducing the specificity of HPV testing Tests should not include noncarcinogenic HPV types, which have no proven independent value in cancer-risk assessment. The group that should not be assayed includes HPV-53, which is much too common and too rarely found in precancer and cancer to be included, and HPV-6 and HPV-11, which cause genital warts.

HPV Test Characteristics III n n n ANY HPV test should have a clinical sensitivity to detect at least ≥ 92% of CIN 3+ The negative predictive value of the test must also be extremely high Data for any HPV test used clinically should be publicly available for peer review. Ideally, independent analysis and documentation should be available for auditing.

HPV Test Characteristics IV n Intra- and inter -batch and inter-laboratory reproducibility for homebrew, manufactured and commercially marketed tests should be documented to ensure reliable performance for use in clinical management. k values of 0. 7 or more for repeat positivity of targeted HPV genotypes (pooled or individual) should be documented to support test robustness

HPV Test Characteristics V n n One limitation of all current tests is the lack of internal standards to evaluate specimen adequacy. Of course, even a standard that measures squamous cellularity for specimen adequacy does not completely ensure that the correct site has been sampled and the cells of interest are represented. WHO HPV DNA standards for HPV 16 and 18 will soon be available
 Cosette wheeler
Cosette wheeler Sum or difference formula
Sum or difference formula National breast and cervical cancer early detection program
National breast and cervical cancer early detection program National breast and cervical cancer early detection program
National breast and cervical cancer early detection program Stage 4 cervical cancer
Stage 4 cervical cancer Hpv cervical cancer
Hpv cervical cancer Hpv cervical cancer
Hpv cervical cancer Cervical cancer hcp
Cervical cancer hcp Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Laprp
Laprp Aims and objectives of curriculum
Aims and objectives of curriculum Sir mortimer wheeler scientific and soil layer method
Sir mortimer wheeler scientific and soil layer method Christa wheeler
Christa wheeler Hipsometro jal
Hipsometro jal Bob wheeler tool time
Bob wheeler tool time Colonel john wheeler middle school
Colonel john wheeler middle school Wheeler de witt
Wheeler de witt